Abstract
Females are more susceptible than males to many autoimmune diseases. The processes causing this phenomenon are incompletely understood. Here, we demonstrate that aged female mice acquire a previously uncharacterized population of B cells that we call age-associated B cells (ABCs) and that these cells express integrin αX chain (CD11c). This unexpected population also appears in young lupus-prone mice. On stimulation, CD11c+ B cells, both from autoimmune-prone and healthy strains of mice, secrete autoantibodies, and depletion of these cells in vivo leads to reduction of autoreactive antibodies, suggesting that the cells might have a direct role in the development of autoimmunity. We have explored factors that contribute to appearance of ABCs and demonstrated that signaling through Toll-like receptor 7 is crucial for development of this B cell population. We were able to detect a similar population of B cells in the peripheral blood of some elderly women with autoimmune disease, suggesting that there may be parallels between the creation of ABC-like cells between mice and humans.
Introduction
The incidence of autoimmune diseases is affected both by genetic polymorphisms and by environmental factors. However, it is well established that autoimmune diseases occur with different frequencies in individuals of different sexes.1-3 Sex could affect the incidence of disease via sex hormones, which certainly affect autoimmunity, because castration of autoimmune-prone NZB/WF1 male mice accelerates the appearance of lupus-like disease in these animals. Likewise, ovariectomized female NZB/WF1 mice given androgens have reduced lupus-like disease.4,5
Although the role of sex hormones in autoimmunity is well established, recent studies have shown that sex-biased autoimmunity is significantly influenced by genes differentially present on sex chromosomes.6,7 To ensure that similar levels of gene products encoded on the X chromosome are expressed in male and female cells, one X chromosome in females is inactivated (lyonized).8 However, lyonization of the X chromosome is not complete, in both mice and humans,9,10 resulting in higher expression levels for some X chromosome–encoded genes in female versus male cells. Indeed, overexpression (which may be < 2-fold) of some of the genes from partially nonlyonized parts of the X chromosome is known to contribute to autoimmune disease in females.6,7
In an attempt to understand how sex affects the immune system, we surveyed the populations of leukocytes in young and old female and male wild-type mice. Although no sex- and age-associated differences were observed in T cells or dendritic cells, a particular population of B cells bearing CD11b and CD11c, but not CD21, was found at a much higher frequency in aged female mice than in young females, or males of any age. Moreover, this population was found at high frequency in young healthy autoimmune-prone mice. This population of B cells secreted autoantibodies on stimulation in vitro. Depletion of these cells in vivo resulted in a reduction of autoreactive antibodies. Development of this B cell population required intact Toll-like receptor 7 (TLR7) signaling, because both MyD88−/− and TLR7−/− old female mice failed to accumulate age-associated B cells (ABCs). We were also able to detect CD11c+ CD21− B cells present with higher frequency in the blood of elderly female autoimmune patients, and in only one healthy individual. Together, these experiments suggest that ABCs may be involved in the production of autoantibodies and may possibly contribute to the sex bias of some autoimmune diseases.
Methods
Isolation of distinct B cell populations
Splenic B cells were purified by negative enrichment using biotinylated TER-119, NK1.1, and anti–T cell receptor αβ antibodies followed by anti-biotin microbeads (Miltenyi Biotec Inc). ABCs were purified with a MoFlo sorter (Dako Colorado Inc) as B220+CD19+CD11b+ to > 95% purity and were verified for CD11c expression. Follicular (FO) B cells were identified as B220+CD19+CD11b−CD21intermediate (int)CD1dint, and marginal zone (MZ) B cells were isolated as B220+CD19+CD11b−CD21highCD1dhigh. To obtain B1 B cells, peritoneal cavity was washed with PBS, and B1 B cells were purified as CD5+B220lowCD19+CD11blow. For analysis, events were collected on a CyAn flow cytometer (Beckman Coulter), and data were analyzed using FlowJo Version 8.8 software (TreeStar Inc).
Chronic immunization and antibody measurement
Two different TLR7 agonists were used for chronic intraperitoneal immunization of C57BL/6 male and female mice: 5 μg of 3M-012 or 50 μg of S-27609 (3M Pharmaceuticals, a gift from R. Kedl). Other TLR agonists were used at the following concentrations: 1 μg of lipopolysaccharide (Escherichia coli O26; B6) and 5 μg of poly(I:C) (InvivoGen). For chronic immunization, mice were immunized intraperitoneally 3 times a week for 2-3 months.
Concentrations of anti-chromatin immunoglobulin G (IgG) antibodies were determined using the protocol of Guth et al.11 For in vitro antibody production, ABCs and MZ, FO, and B1 B cells were incubated at 106 cells/mL in complete Dulbecco modified Eagle medium with or without TLR7 agonist 3M-012 (1 μg/mL). Supernatants were harvested at day 7, and the concentration of total and anti-chromatin IgG was determined by ELISA.
Generation of bone marrow chimeras
Bone marrow cells were isolated from wild-type C57BL/6, CD11c-DTR/GFP, TLR7−/−, or uMT mice. The C57BL/6, CD11c-DTR/GFP, or TLR7−/− bone marrow cells were mixed with bone marrow from uMT mice at 1:4 proportions and 5 × 106 cells were intravenously injected into lethally irradiated (900 rad) B6-Ly5.2 (CD45.1) mice. At least 90% of the B cells in chimeric mice were derived from the donor animals. The total number of donor B cells varied from mouse to mouse, with the minimal number 15 × 106 and the maximal number 70 × 106 per spleen.
DT treatment
For depletion of CD11c+ cells, mice were injected intraperitoneally with 4 ng/g body weight diphtheria toxin ([DT]; in PBS; Sigma-Aldrich). The efficacy of the depletion was examined using flow cytometry at 1, 4, 7, and 18 days after treatment (Figure 7B).
Gene array
Total RNA from at least 500 000 cells from each purified population was extracted using the PicoPure RNA isolation kit (Arcturus), and RNA integrity was assessed using a bioanalyzer (Agilent Technologies). Fragmented, labeled RNA samples were then hybridized overnight onto Affymetrix mouse genome 430 2.0 microarray, containing 45 101 probe sets. Microarray results were analyzed with GeneSpring X (Agilent Technologies). All microarray data are available on the Gene Expression Omnibus under accession GSE28887.
The normalized hybridization intensity for each probe set was calculated using the GC-RMA method implemented in the GeneSpring X software package as the default setting. Genes whose expression was increased (threshold 2.0) within ABCs compared with the other B cell populations were subjected to one-way statistical test, using a Welch t test (parametric test, variances not assumed equal), with a P value cutoff of .05.
Patients and HD controls
Patients were recruited from the faculty practice of 3 board-certified rheumatologists during routine clinical visits at National Jewish Health with the following diagnosis: rheumatoid arthritis ([RA]; n = 26), systemic sclerosis (scleroderma; [SSc]; n = 14), and systemic lupus erythematosus ([SLE]; n = 13). Healthy donors ([HDs]; n = 36) were volunteers working at National Jewish Health. Samples were collected after individuals signed informed consent and Health Insurance Portability and Accountability Act authorization in accordance with National Jewish Health institutional board–approved protocols and the Declaration of Helsinki (supplemental Table 2; available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Peripheral blood mononuclear cells from human heparinized blood samples were purified by density gradient as a buffy coat. Samples were analyzed on a CyAn flow cytometer (Beckman Coulter), and the data were analyzed using FlowJo Version 8.8 software (TreeStar Inc).
Results
CD11c+CD11b+ B cell population accumulates in aged females
Given the fact that females are more susceptible to many autoimmune diseases than males,2 and that the incidence of many autoimmune diseases increases with age,12,13 we performed flow cytometric comparisons of hematopoietic cells from wild-type C57BL/6 mice of either sex at various ages. The analysis showed that the spleens of elderly female C57BL/6 animals contained significantly more CD11b+ CD11c+ cells than the spleens of male mice of the same age or of young mice of either sex (Figure 1A). Closer examination revealed that these cells were B220+, IgM+, CD11b+, CD11c+, and CD19+ (Figure 1A,D); therefore, they were an unexpected and previously undescribed population of B cells, distinguishable from other cells in the spleen. The total number and the frequency of these cells were always higher in elderly female mice than in elderly males or in young mice of either sex (Figure 1B-C). Given that the cells occur in high frequency in aged mice, we named them ABCs. A substantial population of ABCs also was observed in aged female but not male BALB/c mice (supplemental Figure 1A).
Elderly female mice contain an enlarged population of CD19+CD11b+CD11c+ B cells (ABCs). (A) Flow cytometric analysis of total spleen (left set of plots) or splenic B cells (gated as IgM+B220+CD4−CD8−NK1.1−; right set of plots) in young (< 12 weeks old) and elderly (> 1 year old) C57BL/6 mice. Data are representative of > 10 independent analyses. (B-C) Average percent and number of CD19+CD11b+CD11c+ cells in spleen of male (blue) and female (pink) C57BL/6 mice. *P < .01 (Student 2-tailed t test). (D) Flow cytometry of FO B cells (CD19+CD11b−; black) and CD19+CD11b+ B cells (red).
Elderly female mice contain an enlarged population of CD19+CD11b+CD11c+ B cells (ABCs). (A) Flow cytometric analysis of total spleen (left set of plots) or splenic B cells (gated as IgM+B220+CD4−CD8−NK1.1−; right set of plots) in young (< 12 weeks old) and elderly (> 1 year old) C57BL/6 mice. Data are representative of > 10 independent analyses. (B-C) Average percent and number of CD19+CD11b+CD11c+ cells in spleen of male (blue) and female (pink) C57BL/6 mice. *P < .01 (Student 2-tailed t test). (D) Flow cytometry of FO B cells (CD19+CD11b−; black) and CD19+CD11b+ B cells (red).
Further flow cytometric characterization of these cells revealed that they had higher forward and side scatter than FO B cells; were positive for CD5 and CD138; and expressed high levels of Fas, CD80, CD86, CD122, vascular cell adhesion molecule-1, and major histocompatibility complex class II, but they were negative for CD21 (Figure 1D). ABCs also were found in lymph nodes and blood in mice > 2 years old (data not shown), perhaps because of their dissemination from spleen.
As determined by green fluorescent protein (GFP) expression, ABCs from aged female CD11c-DTR/GFP mice indeed expressed CD11c, confirming the specificity of the antibody staining and the unexpected phenotype of these B cells (supplemental Figure 1B).
To check that the cells we detected were not simply a manifestation of nonspecific staining of myeloid cells and to test whether the appearance of ABCs correlated with increases of other splenic populations, we compared the cellular compositions of spleens from young and old female C57BL/6 mice. As shown in supplemental Figure 2, ABCs were clearly a specific population, and apart from ABCs, no apparent differences in percentage or numbers of various spleen populations were detected between old and young female mice.
ABCs appear early in autoimmune-prone mice
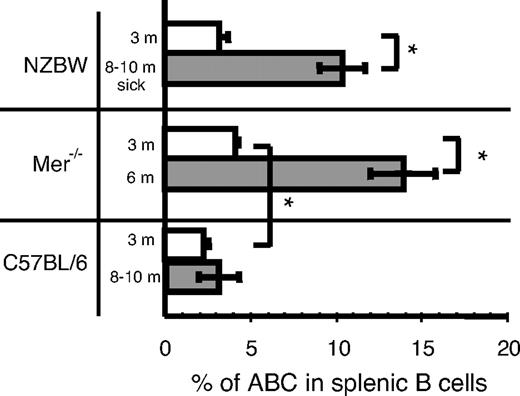
Because many autoimmune diseases affect each sex with different frequencies, we assessed the frequency of ABCs in autoimmune-prone strains at various ages. We performed flow cytometric analysis of spleen cells from 3- and 8- to 10-month-old female NZB/WF1 mice, which develop a lupus-like autoimmune disease with strong similarities to human SLE.14 It is important to note that although 3-month-old mice appeared healthy, all the 8- to 10-month-old mice possessed high level of protein in their urine (data not shown). As shown in Figure 2, 8- to 10-month-old NZB/WF1 mice with ongoing disease had a significant increase in the percentage of ABCs in their spleens compared with age-matched C57BL/6 mice (Figure 2). Also, the absolute number of ABCs in these autoimmune-prone mice was increased proportionally compared with the percentage in age matched C57BL/6 mice (data not shown).
Increased number of ABCs in autoimmune prone mice at the time of onset of autoimmunity. The percentage of ABCs in splenic B cell populations was determined by flow cytometry in female mice of indicated strain and age. Bars represent mean (± SEM) of at least 5 mice per group. *P < .01 (Student 2-tailed t test).
Increased number of ABCs in autoimmune prone mice at the time of onset of autoimmunity. The percentage of ABCs in splenic B cell populations was determined by flow cytometry in female mice of indicated strain and age. Bars represent mean (± SEM) of at least 5 mice per group. *P < .01 (Student 2-tailed t test).
To confirm that these results were not model-specific and could be found in other autoimmune models, we tested Mer−/− mice for the presence of ABCs in the spleen. In mice lacking the tyrosine kinase, Mer (Mer−/−), there is inefficient uptake of apoptotic cells, and these mice develop anti-nuclear antibodies.15 As in NZB/WF1 mice, the appearance of autoantibodies in these mice is accelerated in females and can be detected by 6 months of age.16 Analysis of 3- and 6-month-old Mer−/− mice for the presence of ABCs in spleens revealed that 3-month-old mice had a 2-fold higher frequency of ABCs than age-matched C57BL/6 mice (Figure 2). This was seen even though, at this age, the mice contained no detectable autoantibodies. The percentage of ABCs in Mer−/− mice was dramatically higher in 6-month-old animals and was significantly higher than the percentage of ABCs in 10-month-old C57BL/6 females (Figure 2).
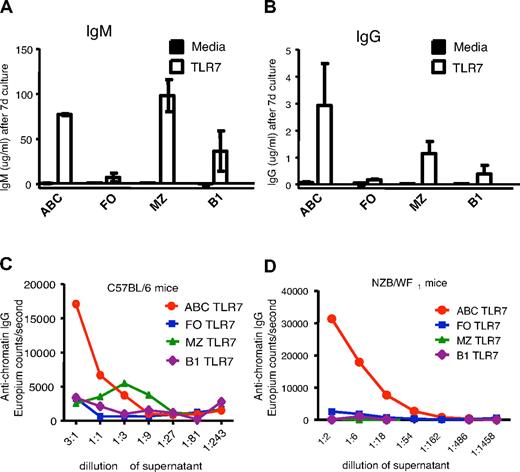
ABCs secrete anti-chromatin IgG autoantibodies in vitro
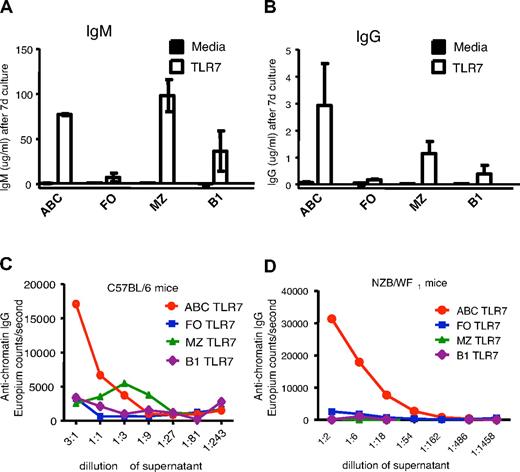
To examine whether ABCs influenced the development of autoimmunity directly, ABCs, FO, MZ, and B1 B cells were isolated from aged wild-type C57BL/6 females and cultured in equal numbers with or without the TLR7 agonist 3M-012,17 a stimulus that is sufficient for B cell activation and antibody production.18 Seven days later, supernatants were analyzed for secreted immunoglobulin M (IgM) and IgG. In the presence of the TLR7 agonist, ABCs and MZ and B1 B cells secreted IgM, with MZ B cells being the highest producers. However, ABCs were the best secretors of IgG in response to the agonist (Figure 3A-B). None of the B cell populations, including the ABCs, secreted Igs when cultured in media alone, suggesting that ABCs are not fully differentiated plasma cells and have to be stimulated to produce antibodies in vitro.
ABCs produce anti-chromatin antibodies on stimulation in vitro. ABCs and FO, MZ, and B1 B cells were isolated from C57BL/6 (A-C) or NZB/WF1 (D) mice and cultured for 7 days in the presence of medium or TLR7 agonist. Total IgM (A), IgG (B), and anti-chromatin IgG (C-D) were subsequently measured in supernatant by enzyme-linked immunosorbent assay. (A-B) Bars represent mean (± SEM) of 3 independent experiments. (C-D) Data are representative of 3 independent experiments.
ABCs produce anti-chromatin antibodies on stimulation in vitro. ABCs and FO, MZ, and B1 B cells were isolated from C57BL/6 (A-C) or NZB/WF1 (D) mice and cultured for 7 days in the presence of medium or TLR7 agonist. Total IgM (A), IgG (B), and anti-chromatin IgG (C-D) were subsequently measured in supernatant by enzyme-linked immunosorbent assay. (A-B) Bars represent mean (± SEM) of 3 independent experiments. (C-D) Data are representative of 3 independent experiments.
The same supernatants were tested for reactivity against chromatin and as shown in Figure 3C, IgG produced by ABCs but not by other B cell populations showed anti-chromatin reactivity. This could have been because of the fact that ABCs secrete more IgG overall than the other types of B cells, thus the ratio of autoreactive to total IgG secreted might have been similar among all the B cells tested. To check this, we measured autoantibody and total IgG levels in immunoglobulin fractions isolated from normal mouse sera. The ratio between the anti-chromatin signal and total IgG in normal serum immunoglobulin was 1/50 μg IgG (data not shown), whereas the ratio of anti-chromatin signal to total IgG in the immunoglobulin secreted by ABCs was ∼ 40/50 μg IgG. Therefore, IgG secreted by ABCs did indeed have a higher relative titer of anti-chromatin antibodies than that secreted by other types of B cells.
To test whether ABCs from autoimmune prone mice also could secrete autoantibodies, B cell populations were sorted from 10-month-old NZB/WF1 female mice with ongoing disease and proteinuria and then stimulated with TLR7 agonist in vitro. Again, only ABCs were able to secrete anti-chromatin IgG, whereas other B cell populations secreted predominantly IgM with no reactivity to chromatin (Figure 3D). In comparing the results from “normal” and NZB/WF1 mice, it is important to note that the supernatants from in vitro experiments were concentrated for detection of the anti-chromatin autoantibodies produced by ABCs from C57BL/6 mice (Figure 3C), whereas the level of the anti-chromatin IgG secreted by ABCs from NZB/WF1 mice was easily detected at a 15-fold dilution (Figure 3D).
To confirm that ABCs could make chromatin-reactive autoantibodies, we made B cell hybridomas from aged C57BL/6 mice. ABCs and FO B cells were isolated from the spleens of 15-month-old C57BL/6 mice. Six of 23 (26%) IgG-secreting hybridoma clones derived from ABCs secreted anti-chromatin IgG, whereas only 2 of the 16 (12.5%) FO-derived IgG-secreting clones showed chromatin reactivity (supplemental Figure 3).
Together, these results demonstrated that ABCs can be the source of autoantibodies and suggest that the increase in the size of the ABC population in autoimmune-susceptible mice might directly influence the onset of autoimmunity.
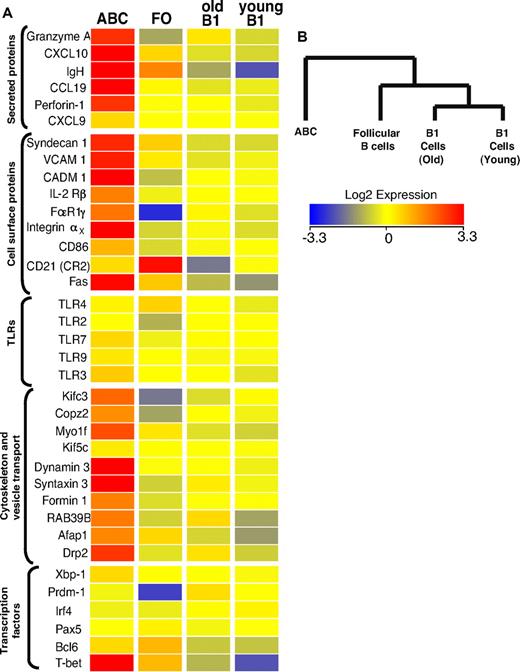
Gene expression profile of ABCs
To characterize the new B cell population more extensively and to compare the properties of ABCs with those of other B cell populations in more detail, gene array analysis was performed. We compared gene expression in ABCs (sorted as B220+CD19+CD11b+ and verified for CD11c expression) and FO B cells (sorted as B220+CD19+CD11b−CD1dintCD21int) purified from the spleens of elderly female C57BL/6 mice and B1 cells (sorted as CD5+B220lowCD19+CD11blow) isolated from the peritoneal cavities of young and old female B6 mice.
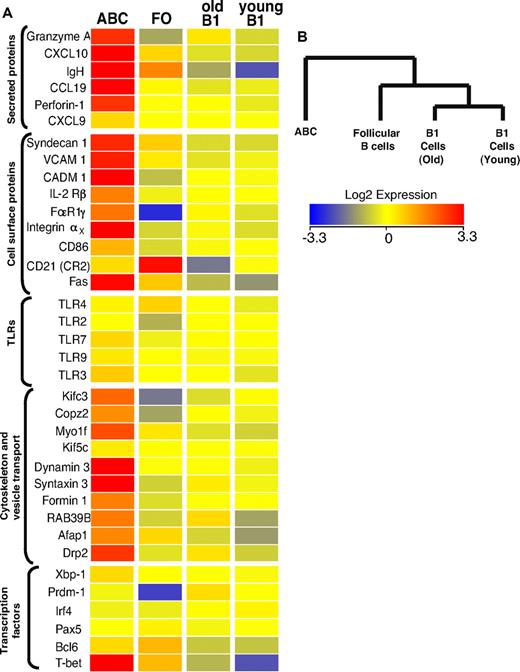
Figure 4A shows a heat map of some of the differentially regulated transcripts, along with that of several control genes that were used to confirm the flow cytometric data. Not surprisingly, CD11c was among the transcripts that were substantially better expressed in the ABC population, in keeping with flow cytometric analysis. Several other strongly up-regulated transcripts, such as those for immunoglobulin heavy chain and Syndecan-1 (CD138), are characteristic of antibody secreting plasma cells. Although Syndecan-1 expression on ABCs was confirmed by flow cytometric analysis (Figure 1D), it was lower than on fully differentiated plasma cells (data not shown). Therefore, combining the Syndecan data with the fact that ABCs do not spontaneously secrete Ig, we think it is likely that ABCs are a unique population of plasmablasts, the precursors of plasma cells, than plasma cells themselves. This is supported by the fact that ABCs express intermediate levels of transcription factors involved in plasma cell differentiation, such as Prdm1, Irf4, and Xbp1 (Figure 4A), indicating that ABCs are more predisposed to become plasmablasts than FO B cells but do not possess plasma cell phenotype yet. CD11c-expressing plasmablasts have been described previously in mice infected with intracellular bacteria.19 The phenotype of these cells is similar to that of ABCs, but not identical. For example, the plasmablasts found in mice infected with bacteria are CD5−CD11bhigh, whereas ABCs are CD5+ and CD11bint (Figure 1D).
Transcriptome analysis of ABCs and FO, MZ, and B1 B cells. (A) Results for some of the genes that changed expression level only in ABCs, together with expression values for some control genes. Up- and down- regulated transcripts are indicated in red and blue, respectively. The magnitude of expression is depicted by the color bar. (B) A genealogical tree, based on gene expression by the different B cell populations that were analyzed, was created by GeneSpring X software based on gene expression profile of analyzed B cell populations.
Transcriptome analysis of ABCs and FO, MZ, and B1 B cells. (A) Results for some of the genes that changed expression level only in ABCs, together with expression values for some control genes. Up- and down- regulated transcripts are indicated in red and blue, respectively. The magnitude of expression is depicted by the color bar. (B) A genealogical tree, based on gene expression by the different B cell populations that were analyzed, was created by GeneSpring X software based on gene expression profile of analyzed B cell populations.
Although ABCs share many surface characteristics with peritoneal B1 cells, the gene array data strongly suggest these 2 cell types are quite distinct (Figure 4B). Furthermore, the expression of ∼ 500 individual genes was greater by > 2-fold between ABCs and all other B cell populations analyzed, again indicating this was a unique B cell population (supplemental Table 1). Together, the flow cytometric and gene profile analysis show that ABCs represent a unique subpopulation of B cells that differ from B1 and FO B cells.
Interestingly, the gene profile analysis also revealed that ABCs express several genes mainly expressed by cells with cytotoxic activity, such as T-bet, perforin, and granzyme A (Figure 4). The expression of these genes was confirmed by flow cytometry (supplemental Figure 1C). The role of these proteins in ABCs is of great interest and will be examined further in future experiments.
TLR7 and myeloid differentiation primary response gene 88 (MyD88) signaling are required for the development of ABCs
To explore which factors might be required for female-specific development of ABCs, we examined young and old female and male mice, deficient for specific genes, for the presence of ABCs in the spleen. First, we tested genes that have been implicated in the development of female-biased autoimmunity.
Considerable evidence suggests that SLE in human and lupus-like disease in mice is accompanied by higher-than-normal levels of IFNαβ in the affected individuals.20-22 Evidence also suggests that the onset of autoimmunity is often associated with disregulation of TLRs, key players of innate immunity involved in the recognition of pathogen-associated molecular structures.23,24
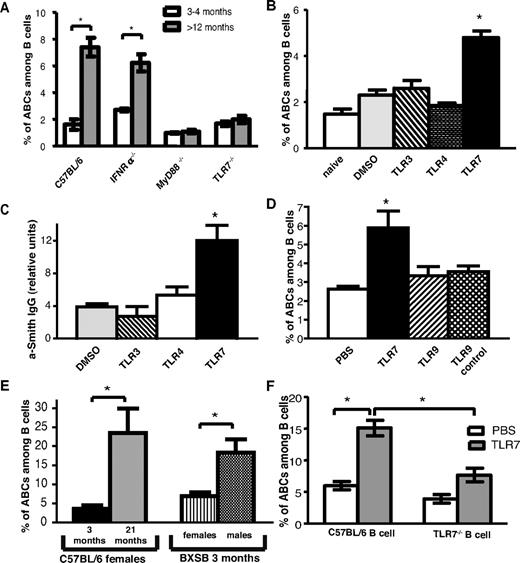
Young and old female mice deficient in the receptor for IFNαβ, IFNαR, or deficient in TLR7 were screened for the presence of ABCs in their spleens (Figure 5A). TLR7−/− aged female mice failed to accumulate ABCs, whereas the number of ABCs in IFNαR−/− mice was similar to that in wild-type C57BL/6 mice. Likewise, ABCs did not accumulate in MyD88−/− mice, which lack the key adaptor to initiate TLR7 signaling (Figure 5A), whereas they were found at normal frequency in TLR3-deficient mice (data not shown).
TLR7 and MyD88 signaling is required for ABC accumulation. (A) Average percentage of ABCs among B cell in spleen of young (12-16 weeks old) and aged (> 12 months old) C57BL/6, IFNR−/−, and TLR7−/−, MyD88−/− female mice. (B) Average percentage of ABCs among B cells in spleen of young (8-12 weeks old) C57BL/6 female mice after 30 immunizations with vehicle or the indicated TLR agonist. (C) Anti-Smith IgG autoantibodies were measured by ELISA in serum of mice chronically injected with TLR agonists. (D) Average percentage of ABCs among B cells in spleen of young (8-12 weeks old) C57BL/6 female mice after 30 immunizations with indicated TLR agonist. (E) Average percentage of ABCs among B cells in spleen of young (12-week-old) BXSB male and female mice. (F) Bone marrow chimeras possessing wild-type or TLR7−/− B cells were treated with vehicle or TLR7 agonist for 2 months, and the percentage of ABCs in the spleen was determined by flow cytometric analysis. Bars represent mean (± SEM) of at least 10 mice per group. *P < .05 (Student 2-tailed t test).
TLR7 and MyD88 signaling is required for ABC accumulation. (A) Average percentage of ABCs among B cell in spleen of young (12-16 weeks old) and aged (> 12 months old) C57BL/6, IFNR−/−, and TLR7−/−, MyD88−/− female mice. (B) Average percentage of ABCs among B cells in spleen of young (8-12 weeks old) C57BL/6 female mice after 30 immunizations with vehicle or the indicated TLR agonist. (C) Anti-Smith IgG autoantibodies were measured by ELISA in serum of mice chronically injected with TLR agonists. (D) Average percentage of ABCs among B cells in spleen of young (8-12 weeks old) C57BL/6 female mice after 30 immunizations with indicated TLR agonist. (E) Average percentage of ABCs among B cells in spleen of young (12-week-old) BXSB male and female mice. (F) Bone marrow chimeras possessing wild-type or TLR7−/− B cells were treated with vehicle or TLR7 agonist for 2 months, and the percentage of ABCs in the spleen was determined by flow cytometric analysis. Bars represent mean (± SEM) of at least 10 mice per group. *P < .05 (Student 2-tailed t test).
That in IFNαR−/− mice the number of ABCs was comparable to that in wild-type mice was quite surprising, considering that one of the main downstream effects of TLR7 signaling is production of IFNαβ.25 This indicates that, for ABC accumulation, TLR7 is acting via a pathway that does not require IFNαβ.
Chronic TLR7 signaling by B cells is sufficient for the induction of ABCs and results in autoantibody production
Because ABCs could not be found in aged TLR7-deficient mice, we hypothesized that chronic signaling through this receptor, but not through other TLRs, is sufficient to induce the accumulation of ABCs. To test this hypothesis, 12-week-old C57BL/6 female mice were injected 3 times a week with low doses of TLR3, TLR4, or TLR7 agonists for 2 months. Then, spleens were examined by flow cytometry for the presence of ABCs. As shown in Figure 5B, TLR7 but not TLR3 or TLR4 stimulation led to the accumulation of ABCs, confirming the unique role of TLR7 in this process.
Because we have shown that ABCs can secrete autoantibodies, we next tested serum from mice chronically treated with TLR agonists for appearance of autoantibodies. In agreement with our hypothesis, anti-Smith autoantibodies were detected in serum of mice treated with TLR7 but not any other TLR agonists (Figure 5C).
Among the intracellular TLRs that recognize nucleic acids, TLR7 and TLR9 have been show to play a critical role in development of such chronic autoimmune diseases as SLE.26 However, chronic stimulation with a TLR9 agonist did not result in accumulation of ABCs, suggesting the unique role of TLR7 signaling in the development of these cells (Figure 5D).
BXSB male mice develop autoantibodies and lupus-like disease because of the translocation to the Y chromosome of the portion of the X chromosome that includes the gene for Tlr7.27 We tested whether the number of ABCs is higher in male versus female BXSB mice. As shown in Figure 5E, at the age of 3 months, male BXSB mice had 3 times more ABCs in their spleens than did females of the same age. These data suggest that ABCs might play a critical role in the progression of the disease.
The gene for TLR7 is encoded on the X-chromosome in mice and injection of TLR7 agonist results in higher IFNαβ production in females than in males.28 This suggests that TLR7 signaling in females is augmented in comparison with males. To test this idea, we compared the accumulation of ABCs, in response to chronic TLR7 stimulation, in the spleens of young C57BL/6 male and female mice. In agreement with a previous study,28 the accumulation of ABCs in response to TLR7 stimulation was significantly higher in female than in male mice (supplemental Figure 4), suggesting unequal expression of this receptor on cells in female and male mice.
To determine whether the accumulation of ABCs on chronic TLR7 stimulation is a result of direct B cell activation or is caused by action of the TLR7 agonist on other hematopoietic cell types such as dendritic cells, bone marrow chimeric mice were created. Lethally irradiated B6-Ly5.1 mice received a mixture of bone marrows from B cell–deficient uMT mice and from either wild-type (C57BL/6) or TLR7−/− mice. As a result, chimeric mice possessed either wild-type or TLR7-deficient B cells, whereas other hematopoietic cell populations included both TLR7-deficient and -sufficient cells. After reconstitution, mice were chronically immunized with TLR7 agonist as described in the experiment shown in Figure 5B and the percentage of ABCs was assessed by flow cytometry. As shown in Figure 5F, ABC development was much reduced in mice lacking TLR7 expression on B cells. In contrast, ABCs appeared in high numbers in mice that contained wild-type B cells, suggesting that TLR7 stimulation must be received by B cells directly for ABC development.
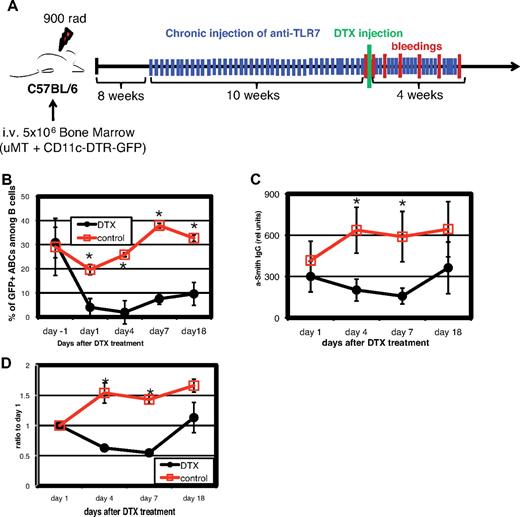
ABCs directly contribute to production of autoantibodies
To demonstrate the role of ABCs in autoimmunity in vivo, we generated bone marrow chimeric mice. Lethally irradiated wild-type mice received a mixture of bone marrow cells from B cell-deficient uMT mice and from CD11c-DTR/GFP mice. The latter mice express DT receptor (DTR) and GFP under the control of the CD11c promoter (Figure 6A). All B cells in such chimeric mice are from the CD11c-DTR/GFP animals, whereas the other hematopoietic cell populations were from both sources of bone marrow (uMT and CD11c-DTR/GFP). After reconstitution, the mice were chronically immunized with TLR7 agonist as described in the experiment shown in Figure 5B and, as expected, developed high titers of anti-Smith autoantibodies. Because the levels of anti-Smith antibodies varied somewhat from mouse to mouse, we assigned the animals to 1 of 2 groups, in which, mouse by mouse, animals in each group were matched for their levels of anti-Smith antibody. One group was then treated with DT. This caused depletion from mice in that group of CD11c+ cells, including ABCs (Figure 6B). As shown in Figure 6C-D, the titer of anti-Smith antibodies in the DT-treated mice was markedly reduced by comparison with controls, both when expressed as absolute titer (Figure 6C) and when expressed on a mouse-by-mouse basis by comparison with the level of anti-Smith antibody before injection of the toxin (Figure 6D). In the DT-treated mice, the anti-Smith titer rose at day 18, before the numbers of ABCs in blood recovered. This might suggest that the ABCs are not involved in anti-Smith antibody production. However, other experiments have shown that, as mice age, ABCs increase in spleen long before they are apparent in blood. Therefore, after DT treatment, ABCs probably reappeared in spleen before blood and thus might have contributed to the rise in anti-Smith antibodies at day 18. Unfortunately, the nature of the protocol of this experiment, with its time course, did not allow us to sacrifice mice and examine their spleens at intermediate time points. Concomitantly, there was a reduction in the total IgG levels in the toxin-treated mice, suggesting that in these chronically TLR7-stimulated animals, a sizeable proportion of the total IgG is produced by ABCs or their recent descendants (data not shown).
Depletion of ABCs reduces amount of autoantibodies in serum. Bone marrow chimeras were constructed as described under “Generation of bone marrow chimeras” such that the only CD11c population that entirely expressed the DTR was that of the ABCs. The chimeras were injected 3 times a week with TLR7 agonist, and 8 to 12 weeks later, they were analyzed for their titer of anti-Smith antibodies. The mice were assigned to control and DT-treated groups by matching mice between the groups for their titers of anti-Smith antibodies and then injected as controls or with DT. (A) Schematic outline of the experiment. (B) Percentage of ABCs in blood before and after injection of DT. (C) Anti-Smith IgG autoantibodies were measured by ELISA in mouse serum at various times after injection of DT. (D) Data presented in Figure 7C are normalized to titers of anti-Smith antibodies of day 1. Bars in panels B through D represent mean (± SEM) of at least 5 mice per group. Data are representative of 2 independent analyses. *P < .05 (Student 2-tailed t test).
Depletion of ABCs reduces amount of autoantibodies in serum. Bone marrow chimeras were constructed as described under “Generation of bone marrow chimeras” such that the only CD11c population that entirely expressed the DTR was that of the ABCs. The chimeras were injected 3 times a week with TLR7 agonist, and 8 to 12 weeks later, they were analyzed for their titer of anti-Smith antibodies. The mice were assigned to control and DT-treated groups by matching mice between the groups for their titers of anti-Smith antibodies and then injected as controls or with DT. (A) Schematic outline of the experiment. (B) Percentage of ABCs in blood before and after injection of DT. (C) Anti-Smith IgG autoantibodies were measured by ELISA in mouse serum at various times after injection of DT. (D) Data presented in Figure 7C are normalized to titers of anti-Smith antibodies of day 1. Bars in panels B through D represent mean (± SEM) of at least 5 mice per group. Data are representative of 2 independent analyses. *P < .05 (Student 2-tailed t test).
These data directly connect the presence of ABCs and autoantibodies. They also suggest a critical role of these cells in the development of autoimmunity, maintenance of autoimmunity, or both.
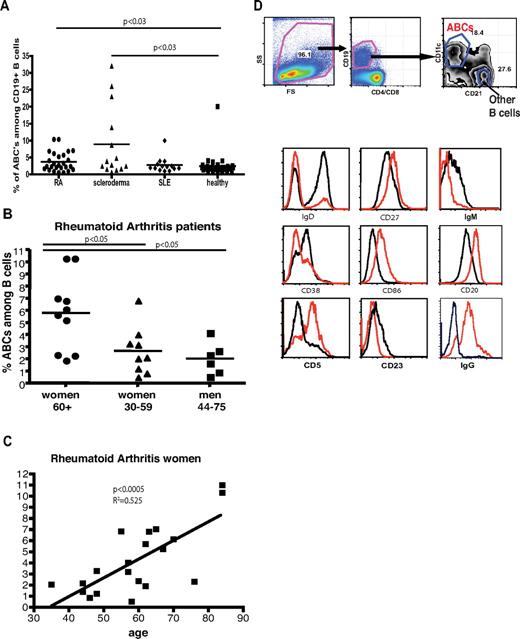
Presence of ABCs in the peripheral blood of autoimmune patients correlates with sex and age
Others studies have described, in autoimmune patients, a population of B cells that seems similar to mouse ABCs. The human B cells in question were identified as CD21−/CD19high and were shown to synthesize anti-chromatin antibodies.29-32 These previous publications showed that only some autoimmune patients contained higher percentages in peripheral blood of the CD21−/CD19high cells, and no mention was made of the sex or age of the patients who contained these cells.
To find out whether the CD21−/CD19high cells were the human analogs of mouse ABCs, we screened peripheral blood samples from patients with RA, SSc, or SLE and from age- and sex-matched HDs for cells that were, like mouse ABCs, CD21−, CD11c+, CD19high. All patients with autoimmune disease were randomly selected from the National Jewish Health rheumatology clinics, and their diagnoses were confirmed by the treating clinician. All subjects were consented for enrollment in accordance with the policies of our institutional review board. We found that the peripheral blood of some patients with RA or SSc contained a significantly higher percentage of ABC-like cells (as defined by the markers listed here). However, an expanded population of ABC-like cells was observed in only one of many SLE patients and only one of many HDs (Figure 7A). Moreover, among RA patients, an expanded population of ABC-like cells was observed in some women with the disease, and these people contain a significantly higher percentage of ABCs in their blood than younger women or men of any age (Figure 7B). Interestingly, for the female RA patients, the percentage of ABCs in blood correlated with the age of the individuals (Figure 7C). This finding might explain why we were not able to detect ABCs in the blood of almost all of the SLE patients we tested. Because SLE is usually diagnosed in younger women and often becomes quiescent after menopause, most of the SLE patients we studied were, although female, relatively young, with the oldest individual tested being 54 years of age.
Presence of ABC-like cells in human peripheral blood. (A) Percentage of ABC-like cells among CD19+ cells in the peripheral blood of patients with RA, SSc, or SLE, or in HDs. Statistical analysis performed using the Mann-Whitney test. (B) Percentage of ABC-like cells among CD19+ cells in peripheral blood of women > 60 years old, women < 60 years old, or men among patients with RA. Statistical analysis performed using the Mann-Whitney test. (C) Correlation of percentage of ABCs among CD19+ B cells in blood with the age of female patients with RA. (D) Phenotypic characterization of human ABCs (red) and other B cells (black). Gating strategy is shown at the top part of the figure.
Presence of ABC-like cells in human peripheral blood. (A) Percentage of ABC-like cells among CD19+ cells in the peripheral blood of patients with RA, SSc, or SLE, or in HDs. Statistical analysis performed using the Mann-Whitney test. (B) Percentage of ABC-like cells among CD19+ cells in peripheral blood of women > 60 years old, women < 60 years old, or men among patients with RA. Statistical analysis performed using the Mann-Whitney test. (C) Correlation of percentage of ABCs among CD19+ B cells in blood with the age of female patients with RA. (D) Phenotypic characterization of human ABCs (red) and other B cells (black). Gating strategy is shown at the top part of the figure.
Further characterization of the ABC-like cells in humans revealed that they are IgD−, IgM−, IgG+, CD38low, CD5high, CD80high, CD86high, CD20high, CD23−, CD27high (Figure 7D). Thus, the cells in humans bore surface markers that were very similar to those that characterize ABCs in mice, with the exception that in mice ABCs are IgD+, IgM+, IgG−.
Thus, we were able to identify B cells in the blood of autoimmune patients who had a phenotype almost identical to that of ABCs in mice. This fact, together with findings of previous reports that cells, from autoimmune patients, with a similar phenotype code for autoantibodies and with the observation that the human B cell population seems significantly more frequent in elderly women, suggests that these cells are the human equivalent of the ABCs we have identified in mice.
Discussion
Here, we demonstrate that an unexpected, CD11c+ B cell population appears at high frequency in spleens of aged female wild-type mice; in the peripheral blood of elderly women with RA or SSc; and, much less frequently, in healthy women. A population of B cells with a very similar phenotype has been described previously in some humans with RA or SLE and in autoimmune individuals with common variable immunodeficiency.29-32 Unfortunately, the sex and age of individuals in whom such cells appeared was not mentioned in these papers, so we cannot be sure that the cells reported there were exactly the same as those described here in mice and humans.
Others studies have reported the existence, in SLE patients, of cells resembling those discussed here.32 By contrast, we found ABC-like cells in only a few of the SLE patients we tested. This difference might have occurred because the duration of the disease of the patients was different between the groups, because it has been reported that the percentage of CD19high/CD21low B cells correlates with the duration of the disease.32 Larger numbers of SLE samples are needed to address the appearance of ABC-like cells during the disease.
B cells bearing CD11c have been described previously. For example, some human B cell cancers, including hairy cell leukemia33 and splenic MZ B cell lymphomas34 express CD11c, as do a subset of nontransformed human memory B cells, which might be involved in protection at mucosa.35 A recent paper has shown that CD11c plasmablasts appear in mice in response to Ehrlichia muris infection.19 The CD11c+ B cells described here are not identical to any of these populations; they are not transformed, do not express a memory phenotype, are mainly found in the spleen, express high levels of CD80 and CD86, and appear spontaneously with age rather than in response to infection.13
That ABCs appear earlier in autoimmune prone mice and are found in humans suffering from autoimmunity suggests that these cells are involved in autoimmune processes. There is also the finding by others and ourselves that only some humans suffering from autoimmune diseases, such as RA, have detectably expanded numbers of ABCs in their peripheral blood, and in the studies shown here, appear only in the blood of elderly women. Does this mean that ABCs occur only late in the course of the disease and are thus the consequence rather than the initiators? The answer to this question is not known; however, it should be kept in mind that, even in mice, ABCs are much more readily detectable in spleen rather than peripheral blood, so women suffering from autoimmune diseases may harbor ABCs in their spleens at a time before these cells appear in blood.
ABCs may not contribute to autoimmunity simply by secreting autoantibody. Their high expression of major histocompatibility complex class II and costimulatory molecules suggests that ABCs also may present self-antigens to T cells and may thus serve to initiate or enhance autoreactivity. Further experiments are required to test the ability of ABCs to present antigen and accelerate autoimmunity by T cell activation.
That virtually all female mice develop increased numbers of ABCs by 15 months of age suggests that their existence depends directly or indirectly on some property peculiar to females. Previous work by others has suggested that TLR7 may be a sex-distinguishing factor. Here, we show that ligands for TLR7, but not TLR3, TLR4, and TLR9, do indeed accelerate the appearance of ABCs.
It is interesting that elderly female mice of strains that are not normally thought to be prone to autoimmune diseases, C57BL/6 and mice strain, accumulate ABCs over the course of time. Moreover, here we show that at least some of the ABCs that appear in these animals secrete IgG autoantibodies. If ABCs are involved in autoimmune disease and can make autoantibodies, why are the C57BL/6 elderly females apparently healthy? Because the results presented here strongly suggest that ABCs in both C57BL/6 and autoimmune prone mice are not anergic, one explanation might be that the increased TLR7 signaling that may occur in females is not sufficient for full differentiation into an autoantibody-secreting cell.
Along these lines, Culton et al36 have suggested that intrinsic antigen stimuli lead to the accumulation of potentially autoreactive preplasmablasts. Their experiments indicate that viral products, such as Epstein-Barr virus-encoded latent membrane protein 2A, enhance the sensitivity of these preplasmablasts to TLR ligands and that TLR ligands can thus activate the cells.36,37 Here, we present a modified version of this idea, in which the somewhat increased level of TLR signaling in females, coupled perhaps with engagement of autoantigens, causes the accumulation of CD11c+ preplasmablasts. Increased TLR7 signaling in normal females is enough to drive the accumulation, but not full activation, of these cells. Some phenomenon in autoimmune mice allows the cells to accumulate more quickly and more frequently convert to plasma cells. That depletion of ABCs leads to a rapid decrease of autoantibodies further confirms that these cells are developmentally close to plasma cells.
Overall, in this report we have demonstrated that a newly discovered population of B cells with a unique phenotype, that of the ABCs, is directly involved in secretion of autoantibodies and thus might contribute to the progression of autoimmunity in mice. We show that this population depends on TLR7 signaling for its appearance, and because TLR7 is encoded on the X chromosome, this may account for the female bias in appearance of ABCs. ABCs may thus provide a clue to the phenomenon of the female bias in occurrence of many autoimmune diseases.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs L. Dragone, T. Detanico, M. MacLeod, and J. White and A. David for assistance. This work was supported in part by U.S. Public Health Service grants AI-18785, AI-22295, AI-52225, and AI-046374.
National Institutes of Health
Authorship
Contribution: A.V.R., K.R., J.W.K., and P.M. designed the experiments; A.V.R. and K.R. performed the experiments and analyzed data; A.F., R.T.M., and J.Z.G. provided patient blood samples; and A.V.R., K.R., and P.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anatoly V. Rubtsov, Department of Immunology, National Jewish Health, 1400 Jackson St, K519, Denver, CO 80206; e-mail: rubtsova@njhealth.org; or Philippa Marrack, Department of Immunology, Howard Hughes Medical Institute, 1400 Jackson St, K512, Denver, CO, 80206; e-mail: marrackp@njhealth.org.