Abstract
Abstract 89
The predictive value of biomarkers for diffuse large B-cell lymphoma (DLBCL) remains controversial especially in the rituximab era. FC-γ receptor 3A (FCGR3A) genotype has emerged as a potential biomarker for rituximab response, supported by the data that antibody-dependent cell-mediated cytotoxicity (ADCC) plays a major role in rituximab-induced cell death. Preclinical data have demonstrated that effector cells homozygous for valine (V) at the rs396991 single nucleotide polymorphisms (SNP) of FCGR3A gene bind the Fc portion of rituximab with significantly higher affinity than those homozygous for phenylalanine (F). In addition, the stronger binding of the V isoform produces more effective ADCC in vitro. Although some recent studies have suggested better clinical outcomes associated with certain FCGR3A genotypes, no published data to date have shown any definitive association between FCGR3A polymorphism and survival outcomes in DLBCL patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). In the present study, we evaluated the predictive value of FCGR3A polymorphism to determine whether FCGR3A genotype could potentially predict survival outcomes in DLBCL patients treated with R-CHOP.
A total of 72 patients with newly diagnosed DLBCL treated at the University of North Carolina, Chapel Hill were analyzed. All patients received R-CHOP chemotherapy. Genomic DNA was isolated from archival formalin-fixed paraffin-embedded tissues and genotyped in the IPIT Molecular Genomics facility at UNC. Using Sequenom iPlex genotyping assays, the genotypes of the rs1801274 (FCGR2A) and rs396991 (FCGR3A) SNP were obtained. For both assays, greater than 90% of the samples produced unambiguous genotypes.
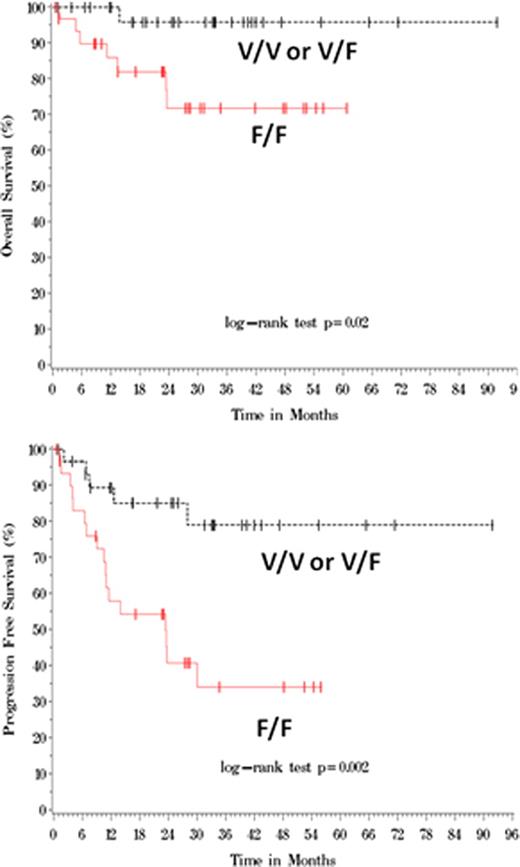
The distribution of the V/V, V/F, and F/F FCGR3A genotypes was 17%, 33%, and 50%, respectively. The frequency of the F/F genotype was somewhat higher in this study population compared to other previously published data. There was no significant difference between the F/F group and the non-F/F (V/V or V/F) group in patient characteristics, including age, race, gender, stage, the International Prognostic Index (IPI) score, LDH, and performance status. Approximately 55 to 60% of the patients had stage III or IV disease. Patients in the F/F group had an overall response rate (ORR) of 73% with a complete response rate (CRR) of 58% while ORR and CRR were 91% and 73%, respectively, in the non-F/F group (p = ns). A significant difference in survival outcome was observed between the F/F and non-F/F patients with a median follow-up of 30 months. According to Kaplan-Meier estimates, overall survival rates (OS) were 72% and 96% in the F/F and non-F/F groups, respectively, at 24 months (Figure 1, p=0.02). Furthermore, the progression-free survival rates (PFS) at 24 months were 41% and 85% in the F/F and non-F/F groups, respectively. (Figure 1, p=0.002). Approximately 59% of the F/F patients relapsed with the median time to progression (TTP) of 30 months while only 15% of the non-F/F patients relapsed with the median TTP not reached during this followup (p=0.01). Multivariable Cox regression analyses were performed to further investigate the predictive value of FCGR3A polymorphism. The IPI score had prognostic impact for OS and PFS as expected, and both the IPI and FCGR3A genotype were independently associated with clinical outcomes. In contrast, FCGR2A polymorphism had no predictive value in the same patient population.
This study suggests that FCGR3A polymorphism has predictive significance independent of IPI in DLBCL patients treated with R-CHOP. FCGR3A genotypes based on F/F versus non-F/F status could potentially be used to predict clinical outcomes in this patient population. A confirmatory analysis is being planned using blood samples from a large prospective trial.
Park:Cephalon: Research Funding; GlaxoSmithKline: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.