Abstract 5271
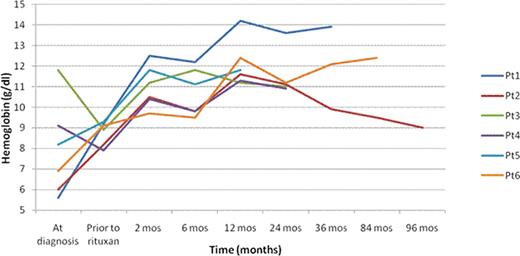
Cold agglutinin disease is an autoimmune hemolytic anemia mediated by cold reactive autoantibodies triggering a complement mediated hemolysis. This condition, when not associated with infection, is characterized by clonal proliferation of CD20+ B cells that produce monoclonal IgM cold agglutinins. Conventional therapies for primary cold agglutinin disease (CAD) are ineffective, but case reports suggest that rituximab, an anti-CD20 monoclonal antibody, may be effective. In this retrospective single institution study, we evaluated the use of rituximab therapy in 6 patients (pts) [1 male, 5 female; median age 70 years (range 62 – 89)]. Three pts had primary CAD, 2 pts had mixed CAD and warm AIHA, and 2 pts had CAD in the setting of CLL. Five pts had received steroid therapy, 2 pts IVIG, 1 pt azathioprine, and 5 pts PRBC transfusion (2–10 units). Five received induction therapy with rituximab 375 mg/m2 IV weekly for four weeks and 1 for seven weeks. Four patients received maintenance rituximab 375 mg/m2 IV every two months (4+ to 12 cycles). All responded to therapy with a median rise in hemoglobin of 1.8 g/dl at 2 months from initiation of induction with further improvement over time (figure 1). Re-induction was performed in 2 pts; both had an initial one year duration of response and both responded following re-treatment. Median duration of response is 3+ yrs (range 1–8+ yrs); the 8+ yr response was in pt 2 who received only induction therapy. Two pts have completed two years of maintenance therapy and remain in remission at 4 months and 2 years post, respectively. Cold agglutinin titers decreased by 4 fold in 2 patients, 1 fold in 1 pt and remained stable in 1. Despite the improvement in hgb in all pts, laboratory evidence of low grade hemolysis persisted in 4 pts. All pts were able to be tapered off steroids and all remain transfusion free. No unexpected adverse events were noted. Rituximab appears to be a well tolerated and effective therapy for cold agglutinin disease. The need for and length of maintenance therapy remains to be determined.
Disclosures:
No relevant conflicts of interest to declare.
© 2011 by The American Society of Hematology
2011