Abstract
Abstract 4986
Splenic marginal zone lymphoma (SMZL) is an uncommon indolent B-cell non-Hodgkin's lymphoma usually presenting with marked splenomegaly and bone marrow (BM) and/or peripheral blood (PB) involvement. Although splenectomy has traditionally been the treatment of choice in symptomatic patients, systemic treatment is required in cases of widespread disease, high risk from surgery, or relapse after splenectomy. There is no standard first line treatment in SMZL, but the anti-CD20 monoclonal antibody Rituximab has shown encouraging results in SMZL, with sustained responses.
To assess, retrospectively, response to treatment, toxicity profile, progression-free survival (PFS) and overall survival (OS) after treatment with rituximab in SMZL.
Twenty-nine patients from two different centers, diagnosed with SMZL between 1982 and 2011, received one or more treatments with rituximab. Eighteen patients (62%) received rituximab alone and 20 (69%) combined with chemotherapy; thus 9 patients received each of these treatments sequentially, to improve response, with or without interim relapse. The median age at diagnosis was 62 years (range 37–89 years); the male:female ratio was 2:3; B symptoms were present in 12 patients; ECOG performance status was 0–2 in 28/29 patients. All presented with splenomegaly, with involvement of the BM in 27 patients, PB in 24, lymph nodes in 11 and extranodal involvement in 7 patients. Diagnosis was made according to the WHO 2008 classification by spleen histology (n=12), BM histology (n=19), PB morphology (n=12) and immunophenotype (n=13). Rituximab monotherapy was administered at 375 mg/m2/weekly x4 weeks. In combination with fludarabine-based regimens (n=14), or other regimens including CHOP (n= 6), rituximab was administered on day 1 of each cycle. Responses were assessed according to the Response Criteria Guidelines for SMZL (Matutes, Leukemia 2008). Toxicity was graded according to the CTCAE v3.0. The Fisher exact test was used to compare best responses between groups. Survival was estimated using the Kaplan-Meier method.
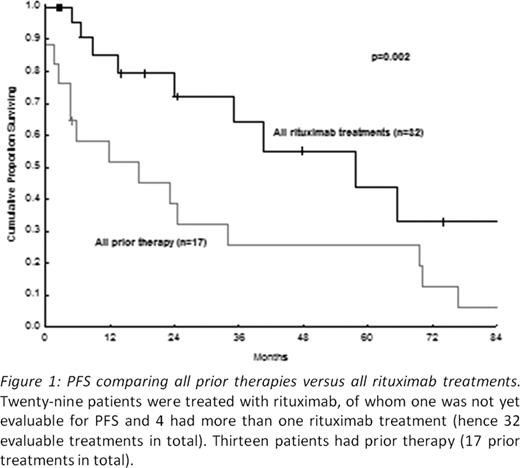
All patients responded to rituximab monotherapy and/or to a combination treatment. At least one complete response (CR) was achieved in 20/29 patients (69%). This compares with a CR in only 4/17 (24%) of these same patients after any prior therapy (p=0.005): 11 patients had received splenectomy, with or without chemotherapy and 6 had received chemotherapy only. There was no difference in the CR rate between rituximab monotherapy (71%) versus rituximab combinations (68%). The most common adverse event was grade 3–4 neutropenia (n=7, 24%). Two patients had grade 3–4 infections. Anemia (n=3) and thrombocytopenia (n=2) were grade 1–2 only. Four cases presented with histological transformation prior to rituximab, all achieving CR with rituximab combination therapy. After a median follow-up of 24 months from rituximab treatment (range 4–102 months), progression-free survival (PFS) has been evaluated in 28/29 patients (1 patient not assessed yet at the time of presenting these data). PFS was longer after rituximab (n=32 evaluable treatments), either alone or in combination, than it had been in these same patients after prior therapy (n=17 prior treatments in total) (p=0.002) (Fig 1). When we compared PFS between rituximab monotherapy (n=17) and rituximab combined with chemotherapy (n=15 combined treatments), single agent rituximab was superior to rituximab in combination (p=0.02) (Fig 2). The median overall survival was not yet reached. Survival at 2 years after rituximab was 100%. Only one death, due to lung cancer, occurred at 48 months follow-up.
In patients with SMZL, the CR rate after rituximab, either alone or in combination with chemotherapy, was significantly better than the CR rate after other prior treatments in the same patients, with manageable toxicity. Although we observed no difference in the CR rate between rituximab monotherapy or combination therapy, PFS was longer in patients receiving rituximab as single agent. Therefore, the addition of chemotherapy did not appear to add any advantage over rituximab alone in our cohort. Rituximab, with or without splenectomy, should be considered in the therapeutic scenario of SMZL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.