Abstract
Abstract 4737
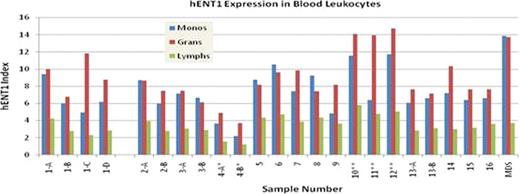
We have developed an assay intended to measure the human Equilibrative Nucleoside Transporter 1 (hENT1) level in leukemic blast cells in blood or bone marrow samples from patients with acute myeloid leukemia or myelodysplasia (MDS). This is ultimately intended as companion diagnostic assay for hENT1 to identify those patients with poor treatment prognosis with cytarabine (ara-C) or other cytotoxic therapies that rely on hENT1 transport into abnormal cells. We optimized an intracellular staining assay incorporating a patent pending technique of utilizing fluorescent labeled monoclonal antibodies, internal fluorescent calibration beads and analytical software that allows identification of leukocyte subpopulations using a novel quantitative flow cytometric assay incorporating antibody binding inhibition by the immunizing peptide to demonstrate specific antigen binding by the anti-hENT1 monoclonal antibody. Initial validation studies were performed on blood samples from both healthy adults and some clinical samples, including samples from HIV+ individuals (samples 4A* and 4B*), infection/sepsis patients (samples 9–11**), and MDS as shown in figures. These preliminary studies showed two novel observations. Firstly expression differs in the major leukocyte populations with expression highest in eosinophils (hENT1 index range of 17.36 – 61.52), followed by neutrophilic granulocytes and monocytes and lymphocytes with the lowest level. Secondly while intra-assay imprecision was low with a CV <7%, some day to day variation was seen in healthy donors and greater variation seen with patient samples, thus indicating significant modulation of cellular expression within myeloid and monocytic cells. These preliminary results suggest retroviral therapy may decrease hENT1 expression, infection or its treatment increases hENT1 expression in myeloid cells, and MDS myeloid cells are also altered in hENT1 levels from the healthy state. These findings indicate potential clinical utility for a quantitative assay of hENT1 expression in hematolymphoid cells in a variety of conditions.
Disclosures:
Davis:Clavis Pharma: Consultancy; Trillium Diagnostics, LLC: Equity Ownership. Curtis:Trillium Diagnostics, LLC: Employment. Booij:Clavis Pharma: Employment. Sandvold:Clavis Pharma: Employment.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011