Abstract
Abstract 4622
Epigenetics is defined by heritable changes in gene expression caused by mechanisms other than changes in the underlying DNA sequence. In vitro or in vivo treatment with DNA methyltransferase inhibitors and/or inhibitors of histone deacetylase (HDAC) have been shown to synergize to transcriptionally activate silenced genes in mantle cell lymphoma. Cladribine, an analogue of the purine adenosine mechanistically has hypomethylating properties including inhibition of methyl group transfer of S-adenosylmethionine, and is FDA approved for hairy cell leukemia (HCL). Combination therapy with cladribine and monoclonal antibodies such as the antiCD20 monoclonal antibody rituximab are under investigation for HCL; either upfront or after relapse post cladribine treatment. One mechanism of resistance to monoclonal antibodies like rituximab can be epigenetic, through transcriptional downregulation of the CD20 receptor gene. Alternatively, CD20 expression may not be downregulated, but other downstream, silenced genes may be epigenetically inactivated. Thus, monoclonal antibody induced antitumor effects, through apoptotic or nonapoptotic cell death or other immunologic mechanisms can be inhibited.
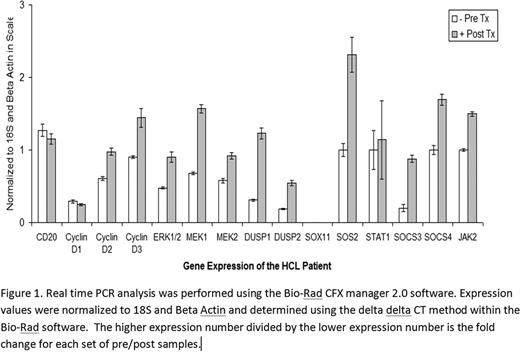
We report a 77 year old caucasian male with HCL, a chronic B-cell lymphoproliferative disorder involving the bone marrow and spleen, without response to cladribine (0.09mg/kg/day continuous infusion Days 1–7) and subsequent therapy with rituximab (375mg/m2 intravenous infusion weekly for 8 weeks). He presented to our institution with lymphocytosis (WBC 50K) and splenomegaly. After informed consent, we initiated combination therapy with cladribine (5mg/m2 intravenous infusion once daily for 5 days), ofatumumab,(300mg week 1, followed by 2000mg weekly for 4 weeks intravenous infusion) and vorinostat, (400mg PO daily for 5 days) and the patient achieved a complete hematologic response(Figure 2). Quantitative reverse transcriptase PCR (RT-PCR) was performed according to the methods described by Liu et al to determine a gene expression pattern for this patient. We were specifically interested in pathways that mediate cell cycle and proliferation. We used RT-PCR to evaluate leukemic HCL cells pre and post therapy for expression of: 1. cell surface antigen CD20, 2. cyclins D1, D2, and D3, proteins which mediate cell cycle progression, 3. members of the MAP Kinase pathway previously identified in the pathogenesis of HCL through constitutive activation, including Mek1 and Mek2, 4. SOX11, a member of a transcription factor encoding a family of genes that play a role in mediating cell differentiation and fate, 5. DUSP1 and DUSP2, the dual specificity phosphatases, and 6. SOS2, and SOCS3, two of the genes from the JAK-STAT signaling pathway.
CD20 expression did not change significantly from pre to post treatment (Figure 1). HCL may over express cyclin D1 without bcl-1 rearrangements or amplification of the cyclin D1 gene. Cyclins D1, D2, and D3 were expressed in this patient with no significant change in regulation measured post treatment. SOX11 was not expressed pre or post treatment (Figure 1). We assayed expression of Mek1, Mek2 and downstream component Erk1/2 pre and post treatment. Mek1 expression was up regulated by 2.3 fold post treatment (Figure 1). Dual specificity phosphatases DUSP1 and DUSP2 act by removing the phosphatases from Mek and Erk, their target substrates. Both DUSP1 and DUSP2 were up regulated post treatment in this patient by 4.0 and 2.9 fold respectively (Figure 1). The JAK-STAT pathway, known for activation of transcription and proliferation, has been implicated in an array of cancers through constitutive activation of STATs. SOS2 and SOCS3, members of this pathway, were up regulated post treatment 2.3 and 4.4 fold respectively in this patient (Figure 1).
We describe a patient with HCL who progressed through cladribine followed by rituximab but achieved a complete hematologic response with the combination of cladrbine, vorinostat, and ofatumumab. We postulate that epigenetic treatment with the combination of a hypomethylating agent and a HDACi enhanced the activity of ofatumumab, without significant changes in expression of CD20. Resistance to monoclonal antibodies may be overcome with epigenetic agents.
Epner:Merck: Consultancy, Honoraria, Speakers Bureau; Novartis: Speakers Bureau; Millenium: Speakers Bureau; Allos: Speakers Bureau; Enzon: Speakers Bureau; GSK: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.