Abstract
Abstract 4449
In our Hospital patients with Chronic Myeloid Leukemia (CML) in chronic phase (CP) were treated with imatinib (Glivec) 400 mg qd from june 2001 to june 2006. Since july 2006, only imatinib copy drugs (brand names: Zeite, Imatec, Imatib, Millatus and Celonib, all of Indian origin) are being used to treat this disease at the Rebagliati Hospital in Lima Peru. We present here the efficacy, security, event-free survival, progression free survival, overall survival of patients treated with imatinib copy drugs.
Retrospective and descriptive analysis of CP-CML patients treated only with imatinib since july 2006. They all initiated the treatment with 400 mg qd. Five year experience is presented here.
We analyzed data from 51 patients (pts) with CP-CML. Median age at diagnosis was 45.1 years (15–90), 49% female and 51% male, all patients were Chromosome Philadelphia positive. Low, Intermediate and High Hasford Score were 32%, 48% and 20%, respectively. Median time from diagnosis to start of imatinib was 7.6 months (0–123). Five patients (9.8%) began treatment more than six months after diagnosis. All patients had at least a 3 month cytogenetic monitoring.
Thirty five (68.6%) and 39 patients (76.4%) achieved Complete Cytogenetic Response (CCyR) and Mayor Cytogenetic Response (MCyR), respectively. Median time to CCyR was 7.6 months and 71.4% of these patients achieved it during the first six months of treatment. CCyR and MCyR were 38% and 67% at 3 months, 57% and 70% at 6 months, 63% and 83% at 12 monhts, 78% and 91% at 18 months, respectively. Quantitative RT-PCR was performed in 22 patients with CCyR and 18 pts (81.8%) had Mayor Molecular Response (MMR) in five years of follow up.
Four pts (7.8%) progressed to Blastic Phase and all of them had Low Hasford Risk at diagnosis. Eight pts (15.6%) changed to 2nd line treatment. Two pts (3.9%) abandoned treatment.
Only 1 pt (1.9%) had both anemias 3–4. Eighteen pts (35.2%) had neutropenia-3, 8 pts (15.6%) had neutropenia-4, 7 pts (13.7%) had thrombocytopenia-3 and 4 pts (7.8%) had thrombocytopenia-4. Neutropenias 3–4 were managed with filgrastim, as treatment and as prophylaxis.
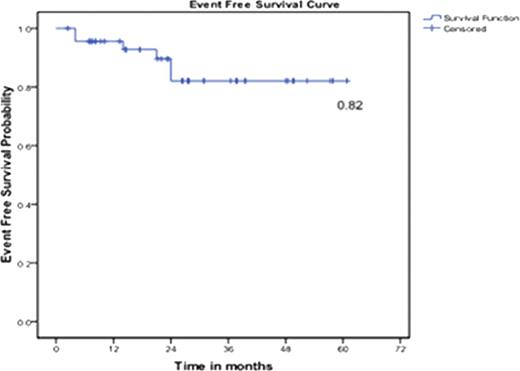
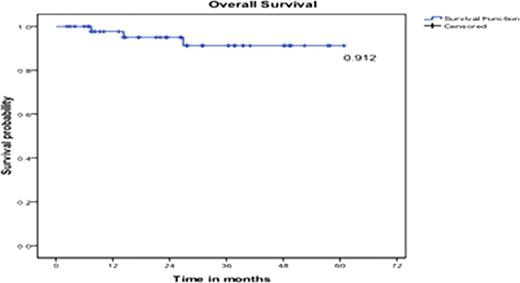
At 5 years, Event Free Survival (EFS), Progression Free Survival (PFS) and Overall Survival (OS) were 82%, 89% and 91%, respectively (Figure 1, 2 and 3).
In our hospital, CP-CML patients treated with imatinib copy drugs obtained important cytogenetic and molecular responses with prolonged EFS, PFS and OS and an acceptable safety profile. Hasford Score was not a good prognosis predictor. Treatment with Imatinib copy drugs has proven to be an acceptable treatment in CML patients in our hospital.
Torres:Bristol Myers Squibb: Speakers Bureau.
Event Free Survival at 5 years (Event: Death, Progression, Loss of MCyR, Loss of CHR)
Event Free Survival at 5 years (Event: Death, Progression, Loss of MCyR, Loss of CHR)
Progression Free Survival at 5 years.
Overall Survival at 5 years.
Author notes
Asterisk with author names denotes non-ASH members.