Abstract
Abstract 365
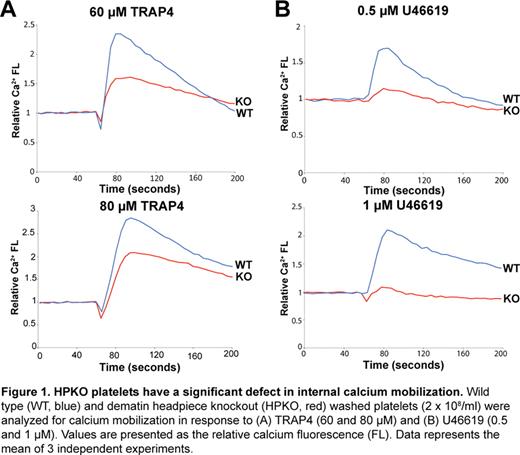
Dematin is an actin binding protein that was originally identified in the human erythrocyte membranes; however, dematin polypeptides are detectable in many non-erythroid cells. In erythrocytes, dematin is located at the spectrin-actin junctions thus linking the skeleton to the plasma membrane. This junctional complex serves to maintain both erythrocyte shape and membrane stability. Dematin is phosphorylated by multiple protein kinases, including the cAMP-dependent protein kinase and protein kinase C, and its actin bundling activity is regulated by phosphorylation. Dematin is also an excellent substrate of calpain-1, a calcium-dependent cysteine protease. Because of the functional similarities between the core components of the membrane skeleton in erythrocytes and platelets, we sought to investigate the physiological function of dematin in platelets. The remodeling of the actin cytoskeleton is known to regulate platelet activation and secretion of platelet granule contents. Western blotting demonstrated abundant expression of the ∼52 kDa polypeptide of dematin in both human and mouse platelets. To evaluate the functional role of dematin in platelets, we utilized the dematin headpiece knockout (HPKO) mouse model previously generated in our laboratory. Headpiece domain of dematin is an actin binding module sharing sequence similarity with the villin-family of cytoskeletal proteins. Importantly, the in vivo deletion of the headpiece domain of dematin resulted in substantial diminution of calcium mobilization in response to multiple agonists of platelet activation (Fig. 1). The reduced calcium mobilization in HPKO platelets was associated with significant inhibition of the platelet aggregation and granule secretion pathways. The HPKO platelets exhibit decreased activation of both the integrin αIIbβ3 receptor and RhoA upon platelet stimulation. Moreover, the HPKO platelets display aberrant morphology upon spreading on the fibrinogen and vWF-coated surfaces. Consistent with these findings, the HPKO mice show a significant clot retraction defect associated with a general tail bleeding phenotype. Mechanistically, the basal level of cAMP remained unaltered in the HPKO platelets suggesting the independence of the observed phenotype upon changes in the cAMP concentration. Immunofluorescence analysis indicated that dematin is associated with two platelet membrane compartments known to be involved in calcium fluxes, i.e., the dense tubular system (DTS) and the plasma membrane. Studies are currently in progress to identify the dematin-associated complex in platelets by Western blotting and proteomics approaches. Together, these results unveil dematin as a novel regulator of calcium homeostasis in platelets with functional implications for the development of new anti-platelet therapies.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011