Abstract
Gains of 1q21 (CKS1B) in plasma cell neoplasms (PCNs) occur frequently and are generally thought to be indicative of an adverse prognosis. The International Myeloma Workshop Consensus Panel 2 (May 2011) found there is insufficient data to suggest routine use of 1q21 (CKS1B) in risk stratification of PCNs. This same Workshop confirmed that FISH testing in PCNs should be plasma cell (PC) specific. Despite this, many laboratories still perform conventional FISH (conv FISH) testing for PCNs primarily due to cost and labor constraints. This study had three objectives: 1) to examine the cytogenetic profile of patients with 1q21 abnormalities, 2) to observe if there was a difference between cytogenetic profiles and the incidence of each additional abnormality detected by cIg FISH vs. conv FISH, 3) to elucidate the significance of 1q21 in the prognosis of PCNs.
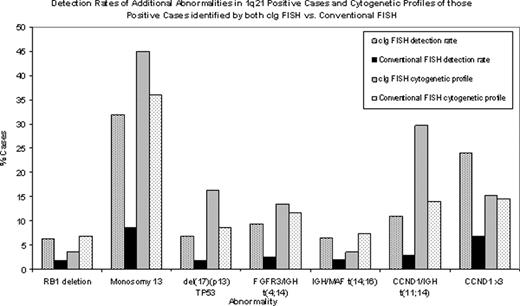
The same probe set was used for both cIg and conv FISH: FGFR3/IGH [t(4;14)], IGH/MAF [t(14;16)], CCND1/IGH [t(11;14)], RB1/LAMP1 (13q14/13q34), TP53 [del(17)(p17.1)] and 1q21 (CKS1B). For conv FISH ≥200 nuclei/probe were scored. For cIg FISH 100 cIg+ cells were scored/probe and ≥25 cIg+ cells required for conclusive reporting. cIg FISH was performed on bone marrow aspirates in 276 cases (age range: 25–91 years); conv FISH 1007 cases (age range: 26–96 years) - all confirmed PCNs. For cIg FISH the %PC range was 0.3–95%; conv FISH 0.02–95%. With cIg FISH there were 246 (90%) abnormal, 14(5%) normal and 16 (5%) inconclusive results. Of the abnormal cases, 111 (45%) had gains of 1q21 (73 (66%) with 3 copies; 38 (34%) with ≥4 copies + [3+≥4 copies]), 127 (46%) had RB1 deletions (RB1−) or monosomy 13 (−13), and 77 (28%) had ‘other abnormalities' (no 1q21 or 13 abnormalities). For conv FISH there were 448 (44.5%) normal and 559 (55.5%) abnormal cases. Of the abnormal cases, 206 (20.5%) had gains of 1q21 (130 (63%) with 3 copies; 76 (37%) with ≥4 copies + [3+≥4 copies]), 240 (24%) RB1− or −13, and 221 (22%) ‘other abnormalities'. True amplification of 1q21 (CKS1B) [≥7–10 copies] was not observed by cIg or conv FISH. The detection rate (% cases) of each aberration occurring in the 111 1q21 positive cases detected by cIg FISH was: −13 31.9%, CCND1x3 23.9%, t(11;14) 10.9%, t(4;14) 9.4%, TP53− 6.9%, t(14;16) 6.5%, and RB1− 6.2%. For conv FISH it was: −13 8.5%, CCND1x3 6.8%, t(11;14) 2.9%, t(4;14) 2.5%, t(14;16) 1.9%, TP53− 1.8%, and RB1− 1.8%. Statistical analysis showed that the detection of additional aberrations in patients with 1q21 gains was significantly higher by cIg FISH compared to conv FISH (<0.0001). For cIg FISH 5.4% of the 1q21 patients had no other aberrations vs. 14.6% by conv FISH. The cytogenetic profile of the 1q21 positive cases identified by both technologies had a similar distribution of aberrations although the detection rates were quite different. The % cases: cIg vs. conv FISH was: t(4;14) 13.5% vs. 11.7%, t(14;16) 3.6% vs. 7.3%, t(11;14) 29.7% vs. 14.1%, TP53− 16.2% vs. 8.7%, RB1− 3.6% vs. 6.8%, −13 45% vs. 35.9%, CCND1x3 15.3% vs. 14.6%.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.