Abstract
Abstract 2561
AML in patients above age 60 is associated with adverse outcomes compared to younger patients. This is due to the higher incidence of adverse risk cytogenetic changes, poor performance status and end organ function that precludes patients from receiving intensive chemotherapy. Large population based studies have reported 5yr survival rates of 5–8% even in patients receiving standard ‘3+7’ induction chemotherapy. Our study looks at the effect of disease and patient characteristics on outcomes in elderly AML patients who received remission induction chemotherapy in the hope of predicting which individuals would benefit most from this treatment.
Retrospective data was collected from 381 patients > age 60 who underwent conventional cytarabine and daunorubicin (7+3) induction and consolidation chemotherapy after clinical evaluation suggesting they were fit for such treatment, from Jan 1990 to Sept 2009.
The follow up duration ranged from 6m–19.5 years. The data collected were age, ECOG performance status,Haematopoetic stem cell transplant comorbidity Index (HCI) (Sorror et al Blood 2005;106:2912),WBC at presentation, bone marrow blast percentage, antecedent hematologic disease (AHD), Cytogenetic risk group by MRC(UK) criteria, remission status, date of relapse, mortality and overall survival (OS). Statistical analysis was performed to determine variables affecting OS using Cox regression analysis. Multivariate Cox regression coefficients were used to generate a nomogram to predict OS based on Akaike's information criterion.
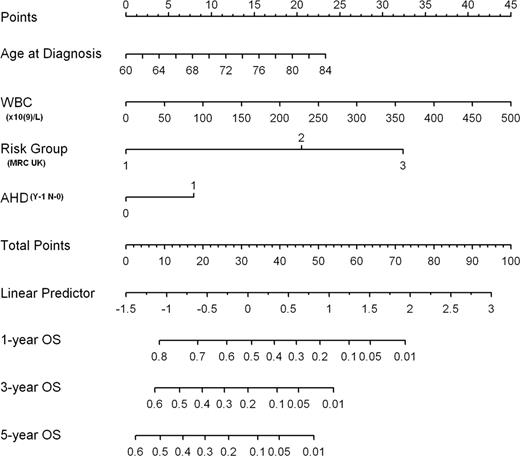
The CR rates in the 3 MRC risk groups were 95%,75% and 40% respectively. The 8 week mortalities in the 3 risk groups 10%,8%and 29% respectively. The 3 month survival was 85%, 1year 50% and 5yr 16% for the patients as a whole. Multivariate analysis showed that age at diagnosis, WBC, cytogenetic risk group and AHD affect OS while sex, ECOG, HCI and BM blast count do not. Using the 4 variable significantly predicting OS a nomogram was developed. Its ability to predict OS of individual patients was evaluated using bootstrapping of a set of 200 resamples. To use the nomogram, draw a line straight upwards to the points axis to determine the number of points received for each of the 4 variables. The sum of these numbers is located on the Total Points axis, and a line is drawn downward to the survival axes to determine the likelihood of 1-, 3- or 5-year OS
AML in patients > age 60 is typically associated with a poor outcome after intensive chemotherapy. However, even among this high risk group results are heterogeneous. This is illustrated in our study where the CR rate and induction mortality varied substantially across cytogenetic risk groups. In addition to the cytogenetic risk group we found age, WBC at diagnosis and the present of AHD to have prognostic value in this elderly group. However, the HCI was not predictive of survival in these AML patients > age 60 receiving standard induction and consolidation chemotherapy. The prognostic patient factors identified in multivariate analysis are easily available in newly-diagnosed AML patients, usually before decisions regarding initial therapy must be made. If confirmed in a larger prospective study, the nomogram we have developed will help clinicians predict the expected survival following intensive chemotherapy, thus helping the patient to make an informed choice regarding risk vs benefit.
Patient Characteristics
| Sex (n) . | Male (212) . | Female (175) . | . | ||
|---|---|---|---|---|---|
| Karyotype (n) | Favorable (29) | Intermediate (197) | Adverse (68) | Unknown (84) | |
| ECOG (n) | 1 (148) | 2 (114) | 3 (10) | 4 (3) | |
| HCI (n) | 0 (169) | 1 (64) | 2 (20) | 3 (35) | 4 (11) |
| Sex (n) . | Male (212) . | Female (175) . | . | ||
|---|---|---|---|---|---|
| Karyotype (n) | Favorable (29) | Intermediate (197) | Adverse (68) | Unknown (84) | |
| ECOG (n) | 1 (148) | 2 (114) | 3 (10) | 4 (3) | |
| HCI (n) | 0 (169) | 1 (64) | 2 (20) | 3 (35) | 4 (11) |
Multivariate Cox Regression Analysis
| Factor . | No of patients . | No of events . | Median survival (months) . | HR (95% CI) . | P-value . | R2 . | Proportion of variation explained . |
|---|---|---|---|---|---|---|---|
| All | 381 | 297 | 12.0 | ||||
| Age | 381 | 297 | 12.0 | 1.047 (1.019, 1.076) | <0.001 | 0.021 | 11.6% |
| WBC | 381 | 297 | 12.0 | 1.004 (1.003, 1.006) | <0.001 | 0.047 | 26.0% |
| Risk Group | 0.107 | 59.1% | |||||
| 1 | 37 | 17 | 92.3 | Reference | |||
| 2 | 263 | 207 | 13.3 | 2.646 (1.591, 4.399) | <0.001 | ||
| 3 | 81 | 73 | 7.8 | 4.642 (2.708, 7.958) | <0.001 | ||
| AHD | 0.015 | 8.3% | |||||
| 0 | 314 | 243 | 12.9 | Reference | |||
| 1 | 67 | 54 | 9.5 | 1.457 (1.073, 1.977) | 0.016 | ||
| Full model | 0.181 |
| Factor . | No of patients . | No of events . | Median survival (months) . | HR (95% CI) . | P-value . | R2 . | Proportion of variation explained . |
|---|---|---|---|---|---|---|---|
| All | 381 | 297 | 12.0 | ||||
| Age | 381 | 297 | 12.0 | 1.047 (1.019, 1.076) | <0.001 | 0.021 | 11.6% |
| WBC | 381 | 297 | 12.0 | 1.004 (1.003, 1.006) | <0.001 | 0.047 | 26.0% |
| Risk Group | 0.107 | 59.1% | |||||
| 1 | 37 | 17 | 92.3 | Reference | |||
| 2 | 263 | 207 | 13.3 | 2.646 (1.591, 4.399) | <0.001 | ||
| 3 | 81 | 73 | 7.8 | 4.642 (2.708, 7.958) | <0.001 | ||
| AHD | 0.015 | 8.3% | |||||
| 0 | 314 | 243 | 12.9 | Reference | |||
| 1 | 67 | 54 | 9.5 | 1.457 (1.073, 1.977) | 0.016 | ||
| Full model | 0.181 |
Sutherland:Centocor Ortho Biotech research & Development: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.