Abstract
The gene for the transcription factor CCAAT/enhancer binding protein α (CEBPA) is important for the differentiation of granulocytes and has been reported to be mutated in patients with acute myeloid leukemia (AML). Double mutation of CEBPA has been shown to be associated with good outcome in the cytogenetic intermediate-risk subgroup. Multiple single nucleotide polymorphisms (SNPs) have been reported in this gene in addition to a 6-nucleotide insertion. Most of the studies of prognostic relevance of CEBPA have been reported in patients treated with standard chemotherapy focusing on the mutations in the CEBPA gene. The prognostic significance of CEBPA is not fully studied in AML patients treated with intensive chemotherapy followed by allogeneic hematopoietic stem cell transplantation (HSCT) in their first complete remission (CR1). We studied the prognostic value of the CEBPA mutations and the SNPs in patients with AML treated with HSCT in CR1.
Samples from 69 patients with AML were analyzed for CEBPA mutations by direct sequencing of the entire coding sequence of the gene. All patients were diagnosed with AML, treated with intensive chemotherapy, then received HSCT in CR1. The group included intermediate (N=42) and adverse (N=27) cytogenetic risk groups.
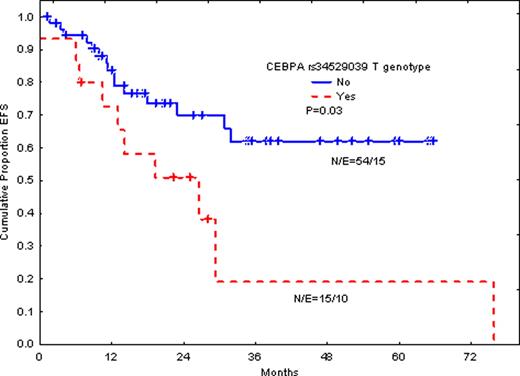
Double CEBPA mutations were detected in 7 patients (10%). However, silent G to T (690g>t) transition in rs34529039 SNP (silent T230) was detected in 15 patients (22%). The same genotype was detected in 17% of normal control individuals. The prevalence of rs34529039 T genotype in our patient population was similar to that detected in the United State population (Quest Diagnostics data: 17% in normal controls and 28% in AML patients). Outcome in patients with double mutations did not differ significantly in event free survival (EFS) or overall survival (OS) in this group of patients, although the number of patients with double mutation is too small. In contrast, the T rs34529039 genotype was significantly associated with shorter EFS (P=0.03) and time-to-relapse (P=0.006). The same trend is seen for OS, although the P-value is not significant due to very few events. When we excluded patients with adverse cytogenetic abnormalities, similar results were obtained and EFS was significantly poor in patients with T phenotype (P=0.04 for EFS).
The data suggests that HSCT after intensive chemotherapy in CR1 may neutralize the prognostic value of CEBPA double mutations. However, the rs34529039 is a very important independent adverse prognostic factor in patients with AML and should be used in stratifying patients.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.