Abstract
There is a paucity of information regarding the factors that affect nonrelapse mortality (NRM) and overall survival among children that develop chronic graft-versus-host disease (cGVHD). We performed multivariate analyses using data from the Center for International Blood and Marrow Transplant Research to identify risk factors for NRM and survival in 1117 pediatric subjects with leukemia or myelodysplastic syndrome, transplanted from related donors, unrelated donors (URD), or unrelated cord blood between 1995 and 2004. We identified 4 variables associated with higher NRM: HLA partially matched or mismatched URD, peripheral blood cell graft, Karnofsky/Lansky score < 80 at cGVHD diagnosis, and platelets < 100 × 109/L at cGVHD diagnosis. Factors associated with significantly worse survival were: age > 10 years, transplantation from HLA partially matched or mismatched URD, advanced disease at transplantation, Karnofsky/Lansky < 80; and platelets < 100 × 109/L. Cumulative incidence of discontinuation of systemic immune suppression at 1, 3, and 5 years after diagnosis of cGVHD were 22% (20%-25%), 34% (31%-37%), and 37% (34%-40%), respectively. This is the largest study elucidating variables affecting outcome after diagnosis of cGVHD in pediatric allograft recipients. These variables may be useful for risk stratification, development of future clinical trials, and family counseling in children with cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) remains the most frequent late complication after allogeneic hematopoietic stem cell transplantation (HSCT), and it represents the major cause of nonrelapse mortality (NRM) and morbidity in long-term survivors.1 Children with cGVHD are of particular interest, given their longer life expectancy and developmental issues compared with adult HSCT recipients. However, pediatric cGVHD remains an understudied area. In adults, the reported incidence of cGVHD ranges between 30% and 50% of HLA-identical sibling transplant recipients,1 whereas in children, the incidence is closer to 25%.2 In a study of unrelated bone marrow transplantation in children and adults, Kernan et al reported a 55% rate of chronic GVHD at one year.3 Factors that increase the likelihood of cGVHD in pediatric allograft recipients include older donor and recipient age, prior grade II to IV acute GVHD, female donors for male recipients, malignant disorder as the transplantation indication, and the use of total body irradiation in transplant conditioning.2 Cord blood as a stem cell source appears to be associated with a decreased incidence of cGVHD.2
Retrospective studies have identified certain risk factors that predict poorer survival in adult patients with cGVHD, such as lichenoid skin changes, extensive skin involvement (> 50% body surface area), elevated bilirubin, progressive onset of cGVHD, thrombocytopenia, gut involvement with cGVHD, low Karnofsky performance status (KPS), and absence of early response to immune suppression. The most consistent factors across these studies are thrombocytopenia at diagnosis and progressive onset of disease.4-11 Risk factors for long-term outcomes in pediatric patients with cGVHD have not been reported. The recovery of the immune system after HSCT in children appears to be different from that of adults.12 For example, in pediatric patients, CD4+ naive cells are often delayed for > 1 year, until thymic activity has resumed.13 This has raised speculation that cGVHD and its modifying factors may be different between adults and children. The increasing use of alternative donors14-16 and peripheral blood (PB) in children17 has led to an increase in the incidence of cGVHD; however, it is unclear whether these factors also affect outcomes once patients develop this complication.
We carried out a large retrospective analysis using the Center for International Blood and Marrow Transplant Research (CIBMTR) database to determine variables significantly associated with survival and NRM in pediatric allograft recipients with a diagnosis of cGVHD. Because this data collection predates the National Institutes of Health Consensus for cGVHD, the older definitions (limited and extensive) were used to capture patients with cGVHD from the CIBMTR database. The goal was to identify clinically applicable transplantation-related and cGVHD-related factors that impact long-term outcome.
Methods
Patient selection
The study population included all pediatric patients (age 0-20 years) who received an allogeneic transplant from a related or unrelated donor (URD) or umbilical cord blood for acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, or myelodysplastic syndrome (MDS) between 1995 and 2004 and were diagnosed with cGVHD (limited or extensive) within one year of transplantation, as reported to the CIBMTR. The CIBMTR is a research organization formed in 2004 through an affiliation between the International Bone Marrow Transplant Registry (IBMTR), the Autologous Blood and Marrow Transplant Registry, and the National Marrow Donor Program (NMDP). The CIBMTR is a voluntary organization involving > 450 transplant centers that have collaborated to share patient data and conduct scientific studies. Participating transplant centers are required to report all consecutive transplantations to a Statistical Center at the Medical College of Wisconsin or NMDP Coordinating Center in Minneapolis. Quality and compliance of data submission are monitored by computerized checks for errors, physician reviews, and on-site audits. Observational studies conducted by CIBMTR are done with a waiver of informed consent and in compliance with Health Insurance Portability and Accountability Act regulations with approval from the Institutional Review Board and Privacy Officer of the Medical College of Wisconsin.
The NMDP retrospectively obtained consent for data submission and study participation from surviving patients or their parent/legal guardian for transplantations it facilitated in the United States during the study period; the NMDP Institutional Review Board waived consent for patients who had died before soliciting consent. To address bias introduced by inclusion of only a proportion of surviving patients (those who consented) but all deceased recipients, a sample of deceased patients was selected using a weighted randomized scheme that adjusts for over-representation of deceased patients in the consented cohort. This weighted randomized scheme was developed based on all survivors in the NMDP database. A logistic regression model was fit to identify factors that predicted whether patients had consented or not consented to use of data collected by the NMDP. This analysis found that the following factors were associated with the likelihood of a patient consenting: age, disease type, race, sex, cytomegalovirus serologic status, and country of transplantation (United States vs non-United States). Using estimated consenting probabilities from this model based on the characteristics of dead patients, a biased coin method of randomization was performed to determine which dead patients were included in the final sample. Thus, this procedure ensures that the preconsented dead patients are included in the sample with the same probability as the survivors who actually consented to participate in the study. Approximately 13% of the surviving patients failed to consent, and 12% of the dead patients were deleted by the weighted randomized method. The afore-described method was tested several times, and on every occasion the proportion of deleted dead patients was similar. Each sample gave essentially the same set of regression parameters and survival estimates.18-20
Study endpoints and definitions
The primary endpoints of the study were NRM and overall survival (OS). NRM was defined as death in continuous remission. OS was estimated from onset of cGVHD. Death from any cause was treated as an event. Surviving patients were censored at the date of last contact. Grade II to IV acute GVHD was graded according to IBMTR criteria based on the pattern of severity of abnormalities in skin, gastrointestinal tract, and liver.21 cGVHD was diagnosed according to Seattle criteria.22 The new National Institutes of Health consensus criteria were not used,23 as they were not available for this retrospective analysis. Disease status at transplantation was classified as early, intermediate, or late. Early disease included patients undergoing HSCT in first remission (acute leukemia) or first chronic phase (chronic myeloid leukemia), or MDS with refractory anemia or refractory anemia with ringed sideroblasts. Intermediate disease was defined as second or later complete remission (acute leukemia), second or later chronic phase/accelerated phase (chronic myeloid leukemia). Advanced disease included patients in relapse or primary induction failure (acute leukemia) or blast crisis (chronic myeloid leukemia), or MDS with refractory anemia with excess blasts or excess blasts in transformation. The NMDP classification of HLA matching status that allows adequate adjustment for donor-recipient HLA compatibility while accounting for best available resolution of typing was used to categorize HLA matching status as well-matched, partially matched, or mismatched. Briefly, well-matched patients had no identified mismatches at HLA-A, -B, -C, and -DRB1 with low/intermediate or high resolution data available at HLA-A, -B and high resolution -DRB1. Partially matched patients had a single locus mismatch at any of the 4 loci and/or missing HLA-C data. Mismatched patients had 2 or more allele or antigen mismatches.24
We defined reduced-intensity/nonmyeloablative regimens as follows: busulfan dose < 9 mg/kg, melphalan dose < 150 mg/m2, and total body irradiation dose < 500 cGy (single or fractionated) or 500-800 cGy (fractionated).19
Statistical analysis
Variables related to patient, disease, and transplant characteristics were described using descriptive statistics. Cumulative incidence for NRM was calculated treating disease progression/relapse as a competing risk.25 OS and 95% confidence intervals (CIs) were calculated using Kaplan-Meier estimates. Patient-related, disease-related, and treatment-related variables were included in the multivariate analyses using a stepwise forward selection technique, and P ≤ .01 was the criterion for inclusion in final models.26 Patient- and transplant-related variables included recipient age, donor type (HLA-matched sibling donor, other related donor, well-matched URD, partially matched URD, and mismatched URD), graft type (bone marrow, PB, and umbilical cord blood), donor-recipient sex mismatch, donor parity, donor-recipient cytomegalovirus serology, conditioning regimen (ablative and reduced-intensity/nonmyeloablative), GVHD prophylaxis, presence and grade of prior acute GVHD, and year of transplantation. Disease-related variables were diagnosis and disease status before transplantation. cGVHD-specific variables included time from HSCT to cGVHD onset, platelet count at cGVHD diagnosis (< 100 × 109/L and ≥ 100 × 109/L), serum bilirubin at cGVHD onset (< 1 mg/dL, 1-2 mg/dL and > 2 mg/dL), type of onset of cGVHD (progressive, interrupted, de novo), and KPS/Lansky score (KPS/L) at diagnosis of cGVHD. Cumulative incidence of discontinuation of immunosuppression was calculated, with discontinuation considered an event and relapse or death treated as the competing risk. Time to discontinuation was calculated as months from diagnosis of cGVHD to the date the patient was last administered treatment of immunosuppression. For children remaining on immunosuppression, the interval was calculated from date of diagnosis of cGVHD to relapse date or date of last contact.
Results
Patient characteristics
Demographic features are summarized in Table 1. There were a total of 1117 patients in this analysis, with a median age of 12 years (range, < 1-19 years). The most common diagnosis was acute lymphoblastic leukemia (49%) followed by acute myeloid leukemia (27%). Thirteen percent of patients had been transplanted with advanced disease, and most patients (97%) had received a myeloablative preparative regimen. Bone marrow was the most common graft source (71%) followed by PB (20%) and umbilical cord blood (9%).
Demographic and clinical features at cGVHD diagnosis are summarized in Table 2. Median onset of cGVHD was 4 months (range, 1-11) after transplantation. Thirty-nine percent of patients had prior grade 3 or 4 acute GVHD. The most common onset for cGVHD was progressive in 49%, interrupted (quiescent) in 28%, and de novo in 19% of the cohort. At time of cGVHD diagnosis, 24% of patients had KPS/L < 80 and 32% had a platelet count < 100 × 109/L. Sixty-one percent of patients needed > 1 year of immunosuppression for cGVHD. The median follow-up of survivors was 72 months (range, 2-162 months) after cGVHD diagnosis.
NRM
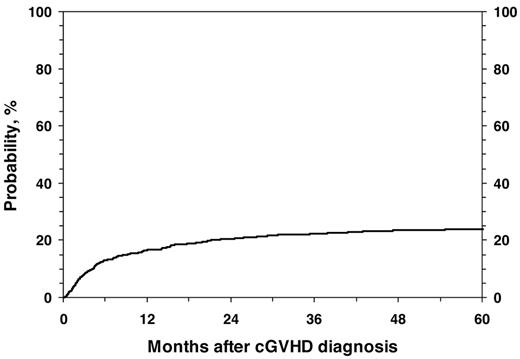
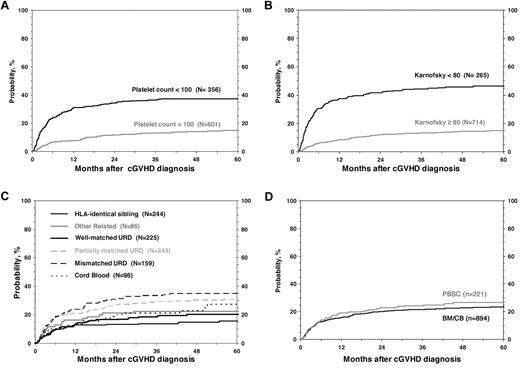
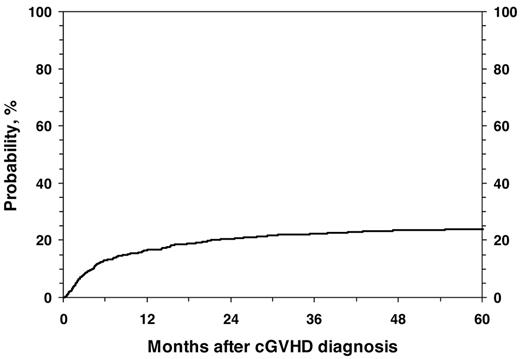
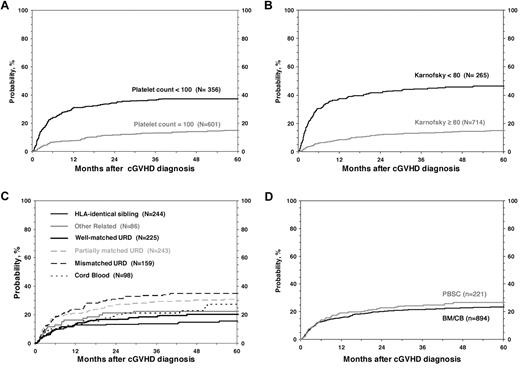
For all 1117 patients, the probability of NRM at 1, 3, and 5 years was 17% (95% CI, 14%-19%), 22% (95% CI, 20%-25%), and 24% (95% CI, 21%-27%; Figure 1). In the univariate analysis, the cumulative incidence of NRM at 5 years was higher for children with KPS/L < 80 (46% vs 15%; P < .001), platelets < 100 × 109/L; (37% vs 15%; P < .001; Figure 2), and for mismatched unrelated donors compared with other donor types (33% vs 22%; P = .003). Multivariate analysis identified 4 factors independently associated (P < .01) with higher NRM: (1) HLA partially matched or mismatched URD, (2) PB stem cell graft, (3) KPS/L < 80 at cGVHD diagnosis, and (4) platelets < 100 × 109/L at cGVHD diagnosis (Table 3).
Cumulative incidence of NRM in 1117 pediatric patients from diagnosis of cGVHD.
Cumulative incidence of NRM according to risk factors included in the multivariate analysis.
Cumulative incidence of NRM according to risk factors included in the multivariate analysis.
Survival
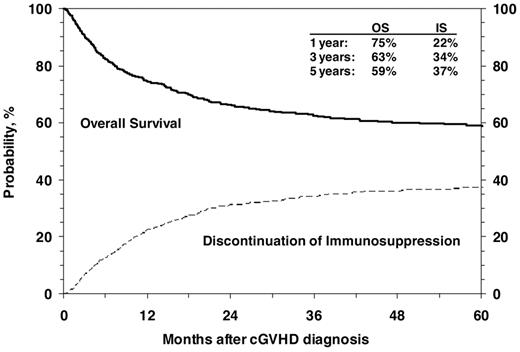
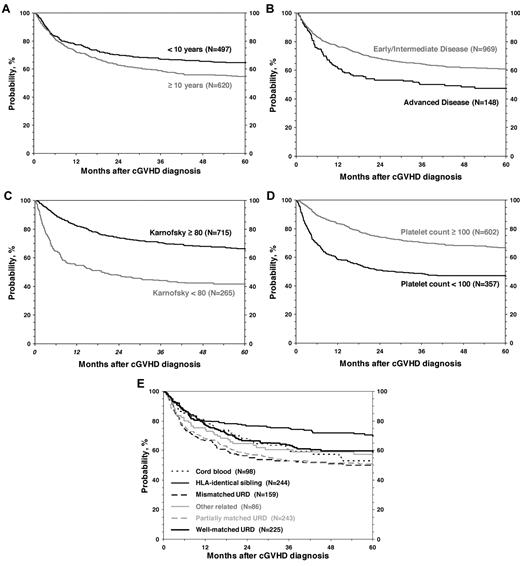
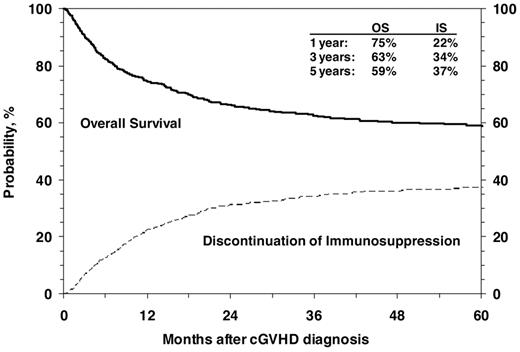
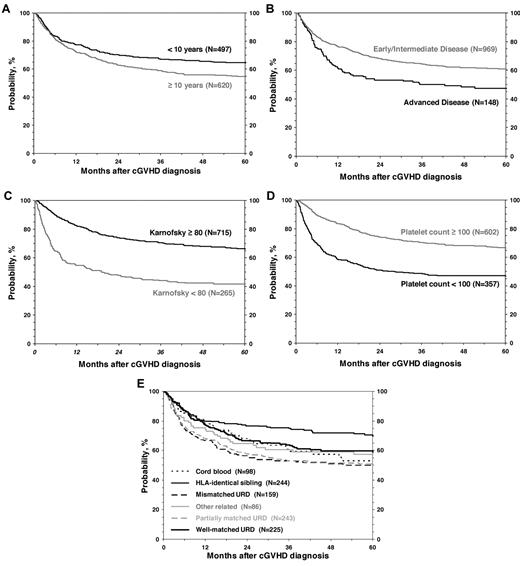
OS after diagnosis of cGVHD at 1, 3 and 5 years was 75% (95% CI, 72%-77%), 63% (95% CI, 60%-66%), and 59% (95% CI, 56%-62%), respectively (Figure 3). Cumulative incidence of discontinuation of systemic immune suppression at 1, 3, and 5 years after diagnosis of cGVHD was 22% (95% CI, 20%-25%), 34% (95% CI, 31%-37%), and 37% (95% CI, 34%-40%; Figure 3). At 5 years, survival was inferior in patients 10 years of age or older (54% vs 65%; P < .001; Figure 4), in patients that had received a partially matched donor (51%) or mismatched URD donor (52%) compared with an other related donor (57%), well-matched unrelated donor (59%), or an HLA-identical sibling (70%; P < .001). Patients that received a transplant for advanced disease had inferior survival (47% vs 61%; P = .003), as did patients with a KPS/L < 80 at cGVHD diagnosis (42% vs 66%; P < .001) and patients with a platelet count < 100 × 109/L at cGVHD diagnosis (47% vs 67%; P < .001). Factors independently associated with worse survival in multivariate analysis were: (1) age > 10 years, (2) transplant from an HLA partially matched or mismatched URD donor, (3) advanced disease at transplantation, (4) KPS/L < 80, and (5) platelets < 100 × 109/L (Table 3). At the time of the study, 660 (59%) of patients were alive. The most common primary cause of death was recurrence of malignancy in 152 (14%), followed by infection (8%) and GVHD in 83 (7%) of patients.
Cumulative incidence of discontinuation of immune suppression after the diagnosis of cGVHD shown with OS.
Cumulative incidence of discontinuation of immune suppression after the diagnosis of cGVHD shown with OS.
Discussion
Although several studies evaluating risk factors for mortality in patients with cGVHD5-11,27 as well as risk factors for the development of cGVHD have been previously reported in adults and children,2,27 risk factors to predict major long-term outcomes in a large cohort of just children with cGVHD have not been studied before. The objective of the current retrospective study was to investigate the risk factors for major outcomes in children diagnosed with cGVHD reported to the CIBMTR. These risk factors could differ from those reported in adults, as different patterns of immune-reconstitution after transplantation have been observed between children and adults.12
In a multivariate analysis, we found 4 risk factors significantly associated with an increased NRM in children with cGVHD: (1) low KPS/L at cGVHD initial diagnosis, (2) thrombocytopenia at cGVHD initial diagnosis, (3) use of partially matched/mismatched unrelated donor, and (4) mobilized PB stem cells as the source of the graft. We also identified 5 risk factors significantly associated with lower OS in the current study: (1) low KPS/L, (2) thrombocytopenia, (3) partially matched/mismatched unrelated donor, (4) older age, and (5) advanced disease at the time of transplantation.
One of the major strengths of this study is the large number of patients, which allowed for identification of significant risk factors associated with major outcomes in children with cGVHD and provided valuable data for risk assessment of children diagnosed with cGVHD.
Interestingly, all risk factors significantly associated with lower OS or with high NRM in children with cGVHD in our study have been identified in adults with cGVHD (ie, thrombocytopenia, low KPS/L at diagnosis of cGVHD, mismatch donors, PB as the source of the graft).5,6,9,27 Because of the constraint of the registry data collected, exploration of other potentially interesting clinical factors unique to the pediatric population could not be considered in our analysis. However, the current study findings support the development of 1 risk stratification or prognostic system that would be simple to use and encompass both children and adults. Such a system could be very useful in the design of clinical trials and for standardization of how we view and treat cGVHD across age groups.
Thrombocytopenia at the time of initial diagnosis of cGVHD has been one of the most consistent risk factors identified to correlate with poor OS. The exact mechanism of thrombocytopenia as a high risk in cGVHD is not well understood, but it could be a surrogate marker for the degree of immune dysregulation in these patients.28 The association between poor performance score and higher NRM is probably multifactorial, as KPS/L may be affected by organ function, nutrition, physical strength, and other factors. A low performance score may also be a reflection of increased inflammation leading to cachexia, elevated resting energy expenditure, and weight loss.29-32
The higher NRM observed in children with cGVHD who receive PB as the stem cell graft source in our study is concordant with previous reports that included adult patients. In previous reports that included adults and children, PB was associated with an increased risk of developing cGVHD but also was associated with a higher degree of cGVHD manifestations and prolonged treatment with immunosuppression compared with recipients of bone marrow grafts.27,33-35 A higher incidence of overall mortality has also been reported in pediatric recipients of PB compared with bone marrow.17 The relationship between PB and higher NRM in pediatric allograft recipients already diagnosed with cGVHD, though, has not been reported. The fact that OS was not decreased by the use of PB in these patients could be because of a greater graft-versus-leukemia effect, which decreases relapse36 and counterbalances the increase in NRM.
The probability of discontinuation of systemic immunosuppression in this study is similar to that reported by the Seattle group, which included both adult and children recipients of related and unrelated donors. In that study, the cumulative incidence of discontinued immunosuppression at 7 years from diagnosis of cGVHD was 50%.35 In this study, more than half of the patients required > 1 year of systemic treatment for control of GVHD. This is a major concern because of the negative impact of immunosuppression on the physical and emotional development of children. The long duration of immunosuppression for control of cGVHD in our study further supports the development of novel therapies for children with cGVHD. Furthermore, it should be used to counsel parents and patients undergoing HSCT.
The current study has several limitations. First, our population predates the National Institutes of Health consensus criteria and therefore may have included some patients with late acute GVHD who were labeled as having cGVHD. Second, the CIBMTR database does not capture percentage of skin involvement or extent of oral involvement at the time of diagnosis of cGVHD, and information related to severity of cGVHD is only recorded as the maximum degree of severity of each affected organ at any time during the previous time interval reported. Thus, we could not examine the extent of skin involvement at diagnosis of cGVHD as a risk factor in NRM or OS, a finding that has been reported to have an impact in other studies.5,6 We were also unable to study the impact of oral involvement in children, which appears to be associated with improved outcomes in adults in some studies.9 Nonetheless, we were able to confirm the most consistent factors at the time of initial diagnosis of cGVHD that have been associated with poor OS and increased NRM, such as thrombocytopenia and low KPS/L score. Both of these risks factors, however, are more objective than clinician measurements of organ involvement by cGVHD, and thrombocytopenia and clinical performance score are more reproducible and easy to obtain; therefore, our results are useful for development of risk stratification. In addition, 49% of our patients had progressive onset of cGVHD. This might represent some patients with manifestations of acute GVHD after day 100. We hence repeated our analysis after excluding patients with early progressive onset cGVHD (those who were diagnosed with progressive onset < 4 months from transplantation). The results of this analysis were similar to the results in the whole population (data not shown). Finally, our data had several missing categories for certain variables, which were included as such in the analysis (categories defined as missing); however, we repeated the analysis after exclusion of incomplete data and found similar results (data not shown). In addition, as the number of patients within the different graft types reported to the registry increases, future analyses that are examining outcomes of cGVHD specifically within cord blood and T cell–depleted graft sources are tremendously important.
This is the largest retrospective study to analyze which factors are associated with a significant risk of NRM after diagnosis of cGVHD in children. Identification of predictors for mortality in children with cGVHD should permit risk-adapted therapy, facilitate planning for future clinical trials, and assist in patient and family counseling. Identification of these risk factors can allow for development and validation of a simple and parsimonious algorithm to assign risk to children at the diagnosis of cGVHD. This algorithm could eventually be used to improve communication with patients diagnosed with cGVHD and their families, to help focus on expectations of available therapy, and to consider participation in clinical trials to improve OS, lower NRM, or shorten duration of administration of corticosteroids and other immunosuppressive agents.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Allos Inc; Amgen Inc; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US Inc; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical Inc; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix GmbH; Children's Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai Inc; Genentech Inc; Genzyme Corporation; Histogenetics Inc; HKS Medical Information Systems; Hospira Inc; Kirin Brewery Co Ltd; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals Inc; Miller Pharmacal Group; Milliman USA Inc; Miltenyi Biotec Inc; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics Inc; Otsuka America Pharmaceutical Inc; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix Inc; StemCyte Inc; StemSoft Software Inc; Sysmex America Inc; THERAKOS Inc; Vidacare Corporation; ViraCor Laboratories; ViroPharma Inc; and Wellpoint Inc.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
National Institutes of Health
Authorship
Contribution: D.A.J., M.A., J.P.K., D.J.W., M.E.F., S.S., M.M.H., and S.Z.P. designed the research; M.A. and A.H. collected data; M.A., J.P.K., and A.H. performed statistical analyses; D.A.J., M.A., J.P.K., D.J.W., A.H., M.E.F., S.S., M.M.H., and S.Z.P. interpreted data; D.A.J. drafted the manuscript; and C.S.C., A.U.-I., B.J.B., J.H.A., M.B., J.-Y.C., M.S.C., R.H.H., L.M.I., T.R.K., S.J.L., E.W.P., R.P.G., H.C.S., S.R.S., and J.R.W. critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David A. Jacobsohn, Division of Blood and Marrow Transplantation Center for Cancer and Blood Disorders, Children's National Medical Center, 111 Michigan Ave NW, Washington, DC 20010; e-mail: dajacobs@cnmc.org.