Abstract
Acute basophilic leukemia (ABL) is a rare subtype of acute leukemia with clinical features and symptoms related to hyperhistaminemia because of excessive growth of basophils. No known recurrent cytogenetic abnormality is associated with this leukemia. Rare cases of t(X;6)(p11;q23) translocation have been described but these were sporadic. We report here 4 cases of ABL with a t(X;6)(p11;q23) translocation occurring in male infants. Because of its location on chromosome 6q23, MYB was a good candidate gene. Our molecular investigations, based on fluorescence in situ hybridization and rapid amplification of cDNA ends, revealed that the translocation generated a MYB-GATA1 fusion gene. Expression of MYB-GATA1 in mouse lineage-negative cells committed them to the granulocyte lineage and blocked at an early stage of differentiation. Taken together, these results establish, for the first time, a link between a recurrent chromosomal translocation and the development of this particular subtype of infant leukemia.
Introduction
Acute myeloid leukemia (AML) accounts for 15%-20% of cases of childhood leukemia.1,2 Acute basophilic leukemia (ABL) is a rare subtype of acute leukemia. Clinical features are close to those of poorly differentiated AML with, in some cases, symptoms related to hyperhistaminemia because of excessive expansion of the basophil compartment.3 The diagnosis of ABL is associated with detection of basophil blast cells. To date, because of the rarity of ABL cases, no recurrent cytogenetic abnormality has been described. Rare cases of t(X;6)(p11;q23) translocations have been reported but these were sporadic.4,5 Of note, a very recent paper reported the cloning of the t(X;6)(p11;q23) in a case of acute monoblastic leukemia.6 We report here 4 cases of ABL with a t(X;6)(p11;q23) translocation occurring in male infants. Patients P1 and P2 have been reported previously.4 Because of its location on chromosome 6q23 and its well established involvement in oncogenesis,7 MYB was a good candidate gene. Our molecular investigations, based on fluorescence in situ hybridization (FISH) and rapid amplification of cDNA ends (RACE), revealed that the translocation generated a MYB-GATA1 fusion gene. This chimera commits myeloid cells to the granulocyte lineage and blocks their differentiation.
Methods
Patients
Characteristics of the patients fit with the diagnosis of ABL (supplemental Methods and supplemental Table 1, available on Blood Web site; see the Supplemental Materials link at the top of the online article).
FISH
Cytogenetic analysis of bone marrow samples was performed by standard methods and FISH as described8 (supplemental Methods, supplemental Figure 1).
Cloning of MYB-GATA1
MYB-GATA1 was isolated using the 5′/3′ RACE Kit (Roche). Full-length MYB-GATA1 cDNA was cloned into the retroviral pMSCVIresEGFP vector (pMIE-MG; supplemental Table 2).
Screening of mutations
The mutational status of FLT3, NPM1, WT1, CEBPa, K-RAS, N-RAS, IDH1, IDH2 was investigated by high-resolution melting and multiplex PCR (supplemental Table 2).
CFC assays
Colony forming cell (CFC) assays were performed on mouse lineage-negative (lin−) cells harvested from C57BL/6 mice, sorted using a Lineage Cell Depletion Kit (Miltenyi Biotec) and transduced with pMIE (control) or pMIE-MG (MYB-GATA1) retroviral vectors. Cells were seeded in triplicate into methylcellulose (M3434; StemCell Technologies) at a density of 5 × 103 cells per 35 mm dishes. In vitro transforming activity was determined by serial replating.9
Long-term cell culture
After 5 platings, cells were cultured in IMDM containing 15% FBS, 100μM β-mercaptoethanol, 10 ng/mL of IL3, 10 ng/mL of IL6 and 50 ng/mL of SCF. After a few weeks, IL6 and SCF were removed.
FACS
Cells were stained with anti–mouse CD11b PE-Cy7, anti–mouse Ly-6G (Gr-1) APC, anti–mouse Fc ϵ Receptor I α (FcϵRIa) PE, and anti–mouse CD117 (c-Kit) PE-Cy5 (eBioscience) and analyzed with a BD FACSCalibur Flow Cytometer (BD Biosciences).
Results and discussion
FISH showed delocalization of the probe spanning the 5′ part of MYB (RP11-104D9) on the derivative chromosome X (Figure 1A). Sequence analysis of a 1.5 kb RACE product revealed in-frame fusion of the 5′ part of MYB to the 3′ part of GATA1 (Figure 1B, supplemental Figure 2). The reciprocal GATA1-MYB fusion was not detected. Because GATA1 is on chromosome X and all patients were males, there was no full-length GATA1 expression. The predicted fusion protein comprises the DNA-binding domain and trans-activation domain of MYB fused to the DNA-binding domain of GATA1 (Figure 1C). Western blotting detected the fusion protein in leukemic cells (supplemental Figure 3A). The nuclear location of MYB-GATA1 was confirmed by transfection experiments (supplemental Figure 3B-C). However, a simple t(X;6) translocation would not result in a functional MYB-GATA1 fusion gene because the 2 genes have opposite genomic orientations. A translocation and an inversion of one fragment, at least, are needed to generate this product. In fact, FISH revealed complex and unbalanced chromosomal rearrangements (supplemental Table 1 and supplemental Figure 4). The mutational status of 8 genes frequently found mutated in AML was tested in 2 patients (supplemental Table 1). P2 displayed a single K-RAS exon 2 (G12S) secondary mutation.
FISH investigation on the MYB gene and structure of MYB-GATA1. (A) Red signal corresponding to RP11-104D9 and spanning the 5′ part of MYB is delocalized on der(X). Localization of BACs on the chromosome 6 is schematized on the left. Normal chromosome 6 (chr.) and derivative chromosomes (der) are indicated. (B) Nucleotide sequence of the fusion mRNA (C) Schematic representation of MYB, GATA1 and MYB-GATA1 proteins. MYB is composed of R1, R2, and R3, which are 3 imperfect 52-residue repeats that encompass the DNA-binding domain (DBD) of MYB. TAD is the transactivation domain of MYB containing a conserved acidic domain and NRD is the negative regulatory domain including a leucine zipper motif (LZ). GATA1 is composed of an activation domain (AD) and 2 zinc finger domains. MYB-GATA1 keeps both the DBD and TAD of MYB and the c-terminal zinc finger domain of GATA1. The breakpoint at the amino acid level is indicated by the black arrow. Of note, the TAD of MYB has lost 9aa (241 to 316) compared with the reference11 (241 to 345) but this portion retains the minimal TAD12 (290 to 315).
FISH investigation on the MYB gene and structure of MYB-GATA1. (A) Red signal corresponding to RP11-104D9 and spanning the 5′ part of MYB is delocalized on der(X). Localization of BACs on the chromosome 6 is schematized on the left. Normal chromosome 6 (chr.) and derivative chromosomes (der) are indicated. (B) Nucleotide sequence of the fusion mRNA (C) Schematic representation of MYB, GATA1 and MYB-GATA1 proteins. MYB is composed of R1, R2, and R3, which are 3 imperfect 52-residue repeats that encompass the DNA-binding domain (DBD) of MYB. TAD is the transactivation domain of MYB containing a conserved acidic domain and NRD is the negative regulatory domain including a leucine zipper motif (LZ). GATA1 is composed of an activation domain (AD) and 2 zinc finger domains. MYB-GATA1 keeps both the DBD and TAD of MYB and the c-terminal zinc finger domain of GATA1. The breakpoint at the amino acid level is indicated by the black arrow. Of note, the TAD of MYB has lost 9aa (241 to 316) compared with the reference11 (241 to 345) but this portion retains the minimal TAD12 (290 to 315).
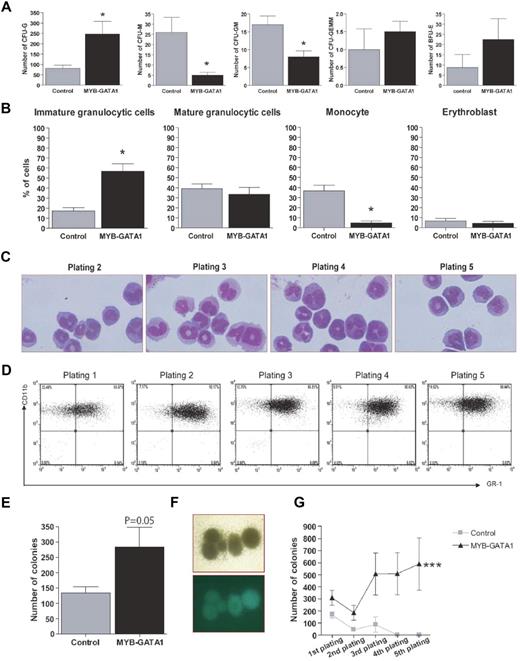
Lin− cells transduced with MYB-GATA1 or the control vector were seeded in methylcellulose containing cytokines (IL6, IL3, SCF and EPO) to compare the formation of myeloid lineage CFUs. On day 12, MYB-GATA1–expressing cells formed significantly more CFU-G (granulocyte), but less CFU-M (monocyte) and CFU-GM (granulomonocyte) colonies than control cells (Figure 2A), suggesting that expression of MYB-GATA1 commits these cells to the granulocytic lineage. A concomitant decrease in the number of monocytes was confirmed (Figure 2B). At the first plating (day 7), the proportion of immature granulocytic cells was higher in cells transduced with MYB-GATA1 than in controls (Figure 2B). From the second plating to the fifth, cells expressing MYB-GATA1 had features of immature granulocytes (Figure 2C) but lacked features typical of basophils (toluidine blue-, FcϵRIa-; supplemental Figure 5 A-C). FACS analysis of these cells through the serial platings showed that they expressed and kept granulocytic markers (Cd11b+/Gr-1+, c-Kit-; Figure 2D). After the fifth plating, cells were propagated in suspension culture for more than 7 months. They remained GFP-positive, Cd11b+/Gr-1+, but the myeloperoxidase test was negative (supplemental Figure 5 D-E). These morphologic and phenotypic characteristics were maintained over time, indicating that MYB-GATA1 is able to block lin− cells at immature stages of granulocytic differentiation. The total number of colonies in CFC assays increased when cells were transduced with MYB-GATA1, in comparison with the control, suggesting an effect on self-renewal of progenitor cells (Figure 2E). Moreover, the CFU-Gs observed in methylcellulose were more dense and compact than normal CFU-G colonies (Figure 2F) suggesting that MYB-GATA1 promotes proliferation of these cells. When the cells were transduced with MYB-GATA1, they continued to form colonies throughout 5 platings (Figure 2G).
Effects of MYB-GATA1 on differentiation, proliferation and survival. (A) Counting of CFUs at day 12. Each graph represents one type of CFU, as indicated. (B) Identification of various types of cells by staining with MGG. Immature granulocytic cells correspond to undifferentiated blasts, myeloblasts, and promyelocytes; mature granulocytic cells correspond to myelocytes, metamyelocytes, and neutrophils. (C) MGG staining of lin− cells expressing MYB-GATA1 in platings 2-5 shows immature cells with discordant morphologic characteristics (basophilic cytoplasm and lobated nuclei with condensed chromatin). Magnification ×630. (D) FACS analysis of cells transduced with MYB-GATA1 and collected from methylcellulose at each plating. Cells remained CD11b+/GR-1+ throughout. Each graph represents the mean values ± SEM of 4 independent assays. (E) Number of colonies counted after 12 days of differentiation in methylcellulose in MYB-GATA1–transduced lin− cells compared with controls. (F) Image of large and dense colonies of CFU-G when cells were transduced with MYB-GATA1. Magnification ×40. (G) In vitro immortalization assay of transduced lin− cells. At each plating, colonies were counted. Cells transduced with MYB-GATA1 continued to form colonies throughout the 5 platings. Statistical analyses were made using paired t test, for counting of CFU, cells and colonies and 2-way ANOVA for in vitro immortalization assay, *P < .05, **P < .005, and ***P < .001.
Effects of MYB-GATA1 on differentiation, proliferation and survival. (A) Counting of CFUs at day 12. Each graph represents one type of CFU, as indicated. (B) Identification of various types of cells by staining with MGG. Immature granulocytic cells correspond to undifferentiated blasts, myeloblasts, and promyelocytes; mature granulocytic cells correspond to myelocytes, metamyelocytes, and neutrophils. (C) MGG staining of lin− cells expressing MYB-GATA1 in platings 2-5 shows immature cells with discordant morphologic characteristics (basophilic cytoplasm and lobated nuclei with condensed chromatin). Magnification ×630. (D) FACS analysis of cells transduced with MYB-GATA1 and collected from methylcellulose at each plating. Cells remained CD11b+/GR-1+ throughout. Each graph represents the mean values ± SEM of 4 independent assays. (E) Number of colonies counted after 12 days of differentiation in methylcellulose in MYB-GATA1–transduced lin− cells compared with controls. (F) Image of large and dense colonies of CFU-G when cells were transduced with MYB-GATA1. Magnification ×40. (G) In vitro immortalization assay of transduced lin− cells. At each plating, colonies were counted. Cells transduced with MYB-GATA1 continued to form colonies throughout the 5 platings. Statistical analyses were made using paired t test, for counting of CFU, cells and colonies and 2-way ANOVA for in vitro immortalization assay, *P < .05, **P < .005, and ***P < .001.
In conclusion, our experiments strongly support the notion that MYB-GATA1 behaves like an oncogene in hematopoietic cells by promoting proliferation of granulocyte precursors and profoundly blocking differentiation in this lineage. The MYB-GATA1 translocation and concomitant loss of full-length GATA1 appear to be the primary events in leukemogenesis. GATA1 has been shown to be a negative regulator of MYB.10 We can assume that, in our patients, the MYB promoter, which drives both MYB and MYB-GATA1 expression, is no longer repressed as GATA1 is disrupted. This hypothesis is supported by the results of RT-qPCR demonstrating overexpression of MYB, which seems to correlate with high expression of MYB-GATA1 (supplemental Figure 6). In a very recent report, Belloni et al6 investigated a case classified as acute monoblastic leukemia carrying the same t(X;6) translocation in a male infant. Like us, they found that this translocation gives rise to a MYB-GATA1 chimeric gene. Hematopoietic reconstitution (with GATA1low lin− cells) revealed that the mice developed either a myelodysplastic syndrome or acute leukemia.6 These data are in line with and complementary to our in vitro studies. In both studies, however, the experiments failed to produce basophil blast cells. This may be explained by additional alterations such as point mutations in other genes or by a specific role of the bone marrow microenvironment, which might be different in patients. The striking similarities between this observation and the 4 patients we reported, strongly suggest that all cases belong to the same clinicopathologic entity, that is, acute basophilic leukemia. Identification of the targets of MYB-GATA1, could shed light on the oncogenic pathways taken by the tumor cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Plateau Technique de Cytométrie en Flux and the Plateau de Séquençage at the Institut Fédératif de Recherche 150, Toulouse, France; the tumor bank of the Center Hospitalier Universitaire Bordeaux, Bordeaux, France and Cindy Cavalier of the Department of Hematology, Center Hospitalier Universitaire Toulouse-Purpan, Toulouse, France. We are indebted to our colleagues of the pediatric oncology departments of the Center Hospitalier Universitaire Toulouse-Purpan and Bordeaux.
Pierre Brousset is supported by the Institut Universitaire de France. Grants were obtained from the Coopération pour l'Investigation Trans-Pyrénéenne de Thérapie Innovante dans les Leucémies network (CITTIL) and l'Association Pour la Recherche Sur le Cancer (no. 4841).
Authorship
Contribution: C.Q. carried out the experiments and wrote the manuscript; E.L. provided samples and designed the experiments; S.S. carried out and interpreted the FISH; C.D. analyzed cell features and performed cell counts; G.S. provided samples and probes; N.P. determined the mutational status of the cases; E.D. designed some experiments (mutational status) and edited the manuscript; C.B. carried out some experiments on cell cultures and retroviral constructs; N.D. provided and described the first 2 cases of ABL and corrected the manuscript; F.X.M. provided patients' samples (P3, P4) and edited the manuscript; and P.B. designed the experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pierre Brousset, MD, PhD, Département de Pathologie, Centre Hospitalier Universitaire Purpan, Place Baylac, 31059 Toulouse Cedex, France; e-mail: brousset.p@chu-toulouse.fr.
References
Author notes
E.L. and S.S. contributed equally to this work.