Hyperlipidemia increases the risk of thrombosis and acute coronary syndromes. In this issue of Blood, Chen et al identify a signaling pathway inside platelets that links oxidized low-density lipoprotein and platelet activation.1
Hyperlipidemia is not only associated with atherosclerosis but also with thrombosis, leading to acute coronary syndromes such as heart attacks. Decades of research have clearly identified the links between elevated plasma lipids and atherosclerosis. Low-density lipoprotein (LDL) deposited in the vessel wall can stimulate inflammation and oxidative stress, generating oxidized LDL (oxLDL).2-4 OxLDL promotes pro-inflammatory responses in monocytes, starting a long process that leads to dysfunctional arteries, fatty streaks, and fibrous plaques.
However, the links between hyperlipidemia and thrombosis are less clear. OxLDL targets platelets, sensitizing them to activating agonists. How? In this issue of Blood, Chen and colleagues now identify a signaling pathway inside platelets that links hyperlipidemia to thrombosis.1
Clues to this connection came from prior observations about the effect of oxLDL on platelets: oxLDL renders platelets more responsive to activation by agonists. This effect is mediated by the scavenger receptor CD36, a transmembrane protein expressed on a variety of cells, including monocytes and platelets.5 As part of the innate immune system, CD36 is a pattern recognition receptor that interacts with oxidized lipoproteins and phospholipids. CD36 is the major receptor for oxLDL. Expressed on monocytes, CD36 mediates macrophage binding and degradation of oxLDL. Expressed on platelets, CD36 mediates oxLDL effects on platelets. CD36 is coupled to a set of distinct signaling pathways, including the MAPK cascade and Src kinases, but the downstream signals inside platelets were unclear.
In the current study, Chen et al explored the effect of oxLDL on platelets.1 They found that oxLDL induces formation of a complex that includes Fyn and Vav1: Fyn is a member of the Src kinase family, and Vav1 is a guanine exchange factor (GEF). Next, they showed that oxLDL triggers Fyn phosphorylation of Vav1. Silverstein and colleagues then compared platelet reactivity between wild-type mice, mice lacking Vav1, and mice lacking Fyn. The research team fed mice a normal or high-fat diet, then collected platelets and measured platelet reactivity. A high-fat diet increased platelet reactivity in wild-type mice, but not in platelets from mice lacking Fyn or Vav1. In a murine model of arterial thrombosis, a high-fat diet increased thrombosis in wild-type mice but not in mice lacking Vav1 and Vav3 isoforms.
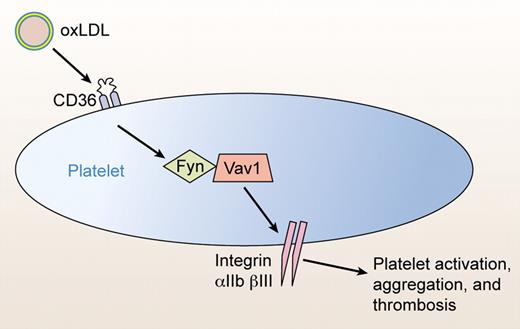
The current study by Chen et al identifies Vav isoforms as new links between oxLDL and platelet activation (see figure). What are Vav proteins?
Vav plays a key role in modulating platelet activation. Hyperlipidemia leads to increased plasma levels of oxidized LDL (oxLDL). OxLDL interacts with the scavenger receptor CD36 on the surface of platelets, triggering an intracellular signaling pathway. The Src kinase Fyn phosphorylates Vav isoforms, which in turn increase the sensitivity of platelets to activation. Vav thus provides a mechanistic link between hyperlipidemia and thrombosis. Professional illustration by Kenneth X. Probst.
Vav plays a key role in modulating platelet activation. Hyperlipidemia leads to increased plasma levels of oxidized LDL (oxLDL). OxLDL interacts with the scavenger receptor CD36 on the surface of platelets, triggering an intracellular signaling pathway. The Src kinase Fyn phosphorylates Vav isoforms, which in turn increase the sensitivity of platelets to activation. Vav thus provides a mechanistic link between hyperlipidemia and thrombosis. Professional illustration by Kenneth X. Probst.
The 3 Vav family members are cytoplasmic guanine exchange factors (GEFs) that couple diverse membrane receptors to intracellular signal pathways.6,7 Vav isoforms are divided into 3 parts: (1) the amino-terminus contains an auto-inhibitory calponin homology domain and an acidic domain; (2) the midsection contains a Dbl homology domain that promotes guanine nucleotide exchange, and a pleckstrin homology domain that binds PI3K products; and (3) the carboxy-terminus contains 2 SH3 domains and 1 SH2 domain, which interact with Vav targets. Nonphosphorylated Vav proteins are auto-inhibited through the Vav calponin homology domain; but during membrane receptor signaling, Vav proteins are phosphorylated, and then bind to downstream Rho GTPases through Vav SH2 and SH3 domains. Each of the 3 Vav isoforms has specificity for different Rho GTPase family members. Vav1 is active in monocyte signaling. Vav1 and Vav3 are important in platelet signaling, but precisely how has remained a mystery.
Prior studies showed that 2 Vav isoforms, Vav1 and Vav3, play an important and redundant role in platelet activation.8-11 Specific agonists such as thrombin, collagen, and thrombopoietin induce Vav1 and Vav3 phosphorylation. Outside-in integrin signaling is coupled to Vav. Vav signaling is mediate by messengers such as such as PLCγ2 and calcium in platelets. Finally, platelets from mice lacking both Vav1 and Vav3 have limited spreading.
Chen and colleagues identify an important role for Vav inside platelets. OxLDL interacts with its CD36 receptor, CD36 triggers Fyn kinase to interact with and phosphorylate Vav1 and Vav3, and Vav1 and 3 then increase platelet sensitivity to agonists.
These observations lead to a set of intriguing questions. Vav proteins increase platelet reactivity in mice fed a high-fat diet, but they decrease platelet reactivity in mice fed a normal chow diet (see Figure 3B-Con page 5744). Does this surprising result imply that some unknown downstream effectors of Vav can inhibit platelet reactivity while others have the opposite effect? If so, are there targets of Vav other than Rho GTPases that regulate the platelet cytoskeleton? For example, does Vav couple with the methyltransferase Ezh2 inside platelets (as it does in T cells), and does methyltransferase activity regulate platelet activation?12 Furthermore, are there endogenous negative regulators of Vav, such as the Cbl ubiquitin ligase, that might limit platelet reactivity?13
Finally, identification of a Vav pathway that modulates reactivity of platelets suggests novel antiplatelet therapies. Drugs that target the Vav pathway might be useful in preventing thrombosis, especially in patients with hyperlipidemia. These significant studies by Chen and colleagues have opened up a new field in platelet research.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■