At least in mice it does. In this issue of Blood, Machlus et al investigated the effects of high fibrinogen levels using a combination of murine models of thrombosis and in vitro studies, to show that high fibrinogen leads to increased formation of thrombi that are more resistant to proteolytic degradation.1
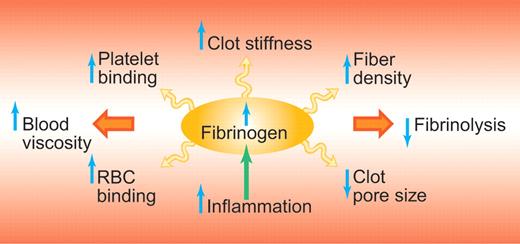
These findings are important because it has long been debated whether elevated fibrinogen causes thrombosis or not. There are clearly a number of mechanisms by which higher levels of fibrinogen could cause thrombosis, including increased blood viscosity, increased fiber density of the fibrin clot, increased resistance of the fibrin clot to fibrinolysis, and altered mechanical properties of the fibrin clot (see figure). While clinical studies consistently have shown elevated levels of fibrinogen in patients with cardiovascular disease and thrombosis, it has not been possible to determine causality of elevated fibrinogen in a clinical study design, which has fueled the debate regarding this issue.2,3
Potential mechanisms by which the risk for thrombosis is increased with elevated fibrinogen. Elevated fibrinogen levels, which are part of the inflammatory response, lead to increases in clot stiffness, increased resistance of the clot to fibrinolysis and increased blood viscosity. RBC indicates red blood cell. Professional illustration by Paulette Dennis.
Potential mechanisms by which the risk for thrombosis is increased with elevated fibrinogen. Elevated fibrinogen levels, which are part of the inflammatory response, lead to increases in clot stiffness, increased resistance of the clot to fibrinolysis and increased blood viscosity. RBC indicates red blood cell. Professional illustration by Paulette Dennis.
Previous in vivo studies have produced contradictory results regarding the role of elevated fibrinogen in thrombosis. Several of these studies were based on a murine transgenic model with raised (murine) fibrinogen (1.5- to 2-fold) levels.4 The fibrinogen used in the study by Machlus and colleagues was of both human and murine origin. Human fibrinogen is known to contain variations that are not present or different from murine fibrinogen, such as the fibrinogen γ prime splice variation, which is unique to human fibrinogen.5 Fibrinogen γ prime has been linked to thrombotic diseases through a number of mechanisms; however, it has also been associated with antithrombotic properties, particularly with regard to venous thrombosis.6 While other methodologic differences might account for some of the discrepancy in the literature, it may be that elevated levels of human fibrinogen in particular increase the risk for certain types of thrombosis.
Fibrin is the main structural component of the thrombus. The conversion of fibrinogen to fibrin by thrombin leads to one of the most remarkable processes in biology in which fibrin spontaneously forms a gel by producing fibers that grow longitudinally as well as laterally, with branch points forming and interconnecting the fibers leading to the formation of a 3-dimensional network that binds platelets and traps other blood cells. Even after the gel point, the architecture of the fibrin network can change quite dramatically.7 Other plasma proteins such as factor XIII, α2-antiplasmin, and fibronectin interact with the fibrin and alter its structural and functional properties. Once the fully organized thrombus is formed, its life span is determined by the resistance of the fibrin clot to proteolysis by plasmin. The generation of plasmin from plasminogen by tissue plasminogen activator is enhanced by the presence of fibrin, particularly after partial degradation by plasmin. The structure of the fibrin clot has been reported to significantly modulate both the rate of proteolysis of fibrin by plasmin as well as the rate of plasmin formation.8
Two previous murine in vivo studies point to an important role of fibrin in the localization and resistance to fragmentation of the thrombus. One study on thrombus composition after laser injury of a cremaster arteriole showed that fibrin is present predominantly at the head of the thrombus and at the center of the platelet plug.9 In areas of the tail of the thrombus that were fibrin poor, small emboli appeared to break off from the thrombus. This potential role of fibrin to stabilize the clot at the site of injury, and thus prevent embolization of the platelet plug, is in agreement with an earlier study of ferric chloride–induced thrombosis in the femoral vessels.10 In this model, 100% of platelet thrombi still formed even with fibrinogen deficiency, but none of these thrombi were located at the site of injury because they embolised to block smaller vessels further downstream.
The difficulty in finding evidence of causality for elevated fibrinogen in clinical studies may be related to inherent methodologic issues of clinical study design and to the complexity of cardiovascular disease. In fact, of all the contributing factors to this disease, only elevated cholesterol and previous occurrence of the disease seem to show consistent associations. This does not exclude causality of other mechanisms. The study by Machlus et al provides clear evidence that higher levels of fibrinogen provide one such mechanism that increases the risk for thrombosis.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■