Abstract
Mutations in bone morphogenetic protein receptor II (BMPR-II) underlie most heritable cases of pulmonary arterial hypertension (PAH). However, less than half the individuals who harbor mutations develop the disease. Interestingly, heterozygous null BMPR-II mice fail to develop PAH unless an additional inflammatory insult is applied, suggesting that BMPR-II plays a fundamental role in dampening inflammatory signals in the pulmonary vasculature. Using static- and flow-based in vitro systems, we demonstrate that BMPR-II maintains the barrier function of the pulmonary artery endothelial monolayer suppressing leukocyte transmigration. Similar findings were also observed in vivo using a murine model with loss of endothelial BMPR-II expression. In vitro, the enhanced transmigration of leukocytes after tumor necrosis factor α or transforming growth factor β1 stimulation was CXCR2 dependent. Our data define how loss of BMPR-II in the endothelial layer of the pulmonary vasculature could lead to a heightened susceptibility to inflammation by promoting the extravasation of leukocytes into the pulmonary artery wall. We speculate that this may be a key mechanism involved in the initiation of the disease in heritable PAH that results from defects in BMPR-II expression.
Introduction
Pulmonary arterial hypertension (PAH) is a severe disease of the small pulmonary arteries characterized by endothelial dysfunction and vascular damage, leading to narrowing of the vessels and, ultimately, right-sided heart failure and death.1 It is now widely appreciated that inflammation is an important pathologically relevant process that is likely to actively contribute to disease initiation and progression by promoting chronic vasoconstriction, remodeling of the pulmonary vessel wall, and thrombosis.1
The endothelial layer in the systemic and pulmonary vasculatures acts as a significant barrier to the underlying tissue, limiting the influx of circulating factors, including inflammatory cells. The process by which inflammatory cells are able to enter tissues from the blood is a tightly regulated process involving multiple steps.2 Defects in several of these processes, as a result of the loss of barrier function, leads to the development of inflammatory lung disorders. For example, elevated lung expression of CCL2 in pulmonary hypertension has been demonstrated to facilitate a recruitment and transmigration of leukocytes through the pulmonary artery endothelium.3 Elevated expression of intercellular adhesion molecule-1 (ICAM-1) mediated by tumor necrosis factor α (TNF-α) has also been described as a mechanism of leukocyte sequestration in the pulmonary microvasculature in patients with acute lung inflammation.4 In addition, subjecting lung microvascular endothelial cells to inflammatory stimuli resulted in a significant loss of plasma membrane expression of key gap junctional molecules, including vascular endothelial (VE)–cadherin, facilitating intercellular gap formation and loss of barrier function.5
Genetic studies have linked germline mutations in gene encoding the transforming growth factor beta (TGF-β) superfamily receptor member, bone morphogenetic protein receptor II (BMPR-II), to underlie the development of heritable forms of idiopathic PAH, encompassing familial and a proportion of sporadic cases of the disease.6 However, less than one-half of individuals who harbor the mutation develop the disease. This fact has led to speculation that the combination of the genetic defect combined with an additional lung-specific environmental trigger may be required for the disease to be manifested.
Multiple studies have been conducted to aid in our understanding of how loss of BMPR-II in the pulmonary vasculature contributes to enhanced vasoconstriction and vessel remodeling.7 In contrast, relatively little work has been undertaken to explore the possible role of BMPR-II in regulating how the pulmonary vasculature controls the local response to an inflammatory insult. Indeed, Hagen and colleagues have conducted the only study that has attempted to define a role for BMPR-II in regulating pathways pertinent to inflammation in pulmonary vascular cells. They demonstrated that BMPR-II exerts a profound ability to suppress the expression of proinflammatory cytokines, such as interleukin-6 (IL-6) in pulmonary artery smooth muscle cells.8 Additional animal studies using BMPR-II heterozygous null mice reveal that these animals do not exhibit an overt pulmonary hypertensive phenotype unless an additional inflammatory insult is applied.9 A recent report has also described the appearance of significant numbers of inflammatory cells in the lung tissue of mice that have been genetically manipulated to be homozygous null for BMPR-II expression using an inducible, endothelial-specific Cre-Lox approach.10 These studies imply that loss of BMPR-II in the endothelium may significantly compromise the ability of the pulmonary vasculature to effectively deal with an inflammatory burden.
In this study, we attempted to define the role of BMPR-II in regulating the barrier function of pulmonary artery endothelial cells in response to 2 pathologically relevant ligands, TGF-β1 and TNF-α, and whose downstream pathways induce pulmonary vascular leakage.11-14 We demonstrate that the reduction of BMPR-II leads to the elevated secretion of the proinflammatory cytokine, IL-8, in response to TGF-β1. Pulmonary artery endothelial-expressed BMPR-II also appears to play a significant role in the maintenance of the barrier function of the monolayer, as evidenced by increased permeability after the loss of BMPR-II. Using static- and flow-induced in vitro systems to assess leukocyte transmigration, loss of BMPR-II facilitates the transmigratory capacity of mononuclear cells and neutrophils after TGF-β1 stimulation and neutrophils after TNF-α stimulation. This facilitated transmigration of neutrophils after loss of BMPR-II appears to be mediated by CXCR2. Using a genetic mouse model with endothelial-restricted expression of BMPR-II, we also demonstrated that the barrier function of the endothelium in the lung is compromised, as evidenced by increased vascular permeability and increased infiltration of leukocytes.
Taken together, our data imply that BMPR-II represents a significant anti-inflammatory molecule in the pulmonary endothelium and provides mechanistic insights into how loss of BMPR-II may prime the pulmonary vasculature of affected individuals to be more susceptible to inflammation-induced tissue damage.
Methods
Isolation of human leukocytes
Venous blood was taken from healthy volunteers and transferred into tubes containing K2EDTA (ethylenediaminetetraacetic acid; 1.6 mg/mL final concentration; Sarstedt). Leukocytes were isolated using a 2-step density gradient as described by Rainger and colleagues.15 The neutrophil or mononuclear cell band was removed from the density interface and washed twice in phosphate-buffered saline (PBS; Sigma-Aldrich), supplemented with 0.15% (wt/vol) bovine serum albumin (BSA; Fraction IV; Sigma-Aldrich), hereafter referred to as PBSA. Neutrophil and mononuclear cell concentrations were determined using a Vi-Cell Series cell viability analyzer (Beckman Coulter) and adjusted to a final concentration of 1 × 106 viable cells/mL.
Endothelial cell culture
Human pulmonary artery endothelial cells (HPAECs) were obtained from Promocell and maintained in endothelial cell growth medium (EGM), supplemented with 2% (vol/vol) fetal calf serum (FCS), 0.4% (vol/vol) endothelial cell growth supplement, 0.1 ng/mL epidermal growth factor, 1 ng/mL basic fibroblast growth factor, 22.5 μg/mL heparin and 1 μg/mL hydrocortisone (complete medium; all from Promocell) until confluent. HPAECs were used between passages 5 through 9.
HPAECs were dissociated from flasks using trypsin/EDTA (Gibco) and seeded onto uncoated, low-density, 3.0-μm pore polycarbonate Transwell filters, which were positioned into matching 24- or 6-well plates (BD Pharmingen) or Ibidi VI slides (Ibidi), which were precoated with 0.2 ug/mL fibronectin (Sigma-Aldrich) in PBS overnight. Seeding density was chosen to yield confluent monolayers in Ibidi slides within 2 hours or filters within 24 hours. Each experiment used HPAECs isolated from a different donor.
Prior to the assay, HPAECs were unstimulated or stimulated with 1 ng/mL human TNF-α (R&D Systems) for 4 hours or 2 ng/mL human TGF-β1 (Peprotech) for 24 hours.
Transfection of HPAECs with gene-silencing BMPR-II siRNA
For BMPR-II silencing, HPAECs were transfected using a scrambled nontargeting control small-interfering (si)RNA (NTCsi) or siRNA targeting human BMPR-II (ON-TARGETplus SMARTpool; Dharmacon). ON-TARGETplus SMARTpool consisted of 4 pooled siRNAs targeting BMPR-II or 4 pooled nontargeting siRNAs. The day before transfection, HPAECs were seeded to yield approximately 60% confluency in 6- or 12-well plates in serum and antibiotic-free EC growth medium. Lipofectamine 2000 (Invitrogen)–RNA complexes were made, as per manufacturers instructions, in Optimem-I (Invitrogen) and added dropwise to each well. HPAECs were incubated with lipid-RNA complexes for approximately 5 hours, after which transfection medium was replaced with complete medium, and the cells were cultured for a further 48 hours.
Western blotting
HPAECs were treated with NTCsi or BMPR-II siRNA, and the cells were harvested with dissociation buffer as described in the paragraph above. After centrifugation at 500g for 5 minutes at 4°C, cell pellets were lysed in 100 μL of ice-cold radioimmunoprecipitation assay buffer (Sigma-Aldrich), containing a phosphatase-inhibitor cocktail (phosSTOP; Roche) and a protease-inhibitor mixture (Complete; Roche). Cell lysates (100 μg of total protein) were separated on 12% (wt/vol) reducing sodium dodecyl sulfate polyacrylamide electrophoresis gels and proteins transferred to a nitrocellulose membrane. Membranes were then blocked with Odyssey blocking buffer (LI-COR Biosciences) and probed with murine anti–BMPR-II antibody (BD Biosciences) overnight at 4°C. Blots were then incubated with an infrared-labeled goat anti–mouse secondary antibody (IRDye 800CW; LI-COR Biosciences) for 2 hours at room temperature. Bands were visualized using the Odyssey Infrared Imaging System (LI-COR). All blots were reprobed with anti–human glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Sigma-Aldrich) to confirm equal loading.
qRT-PCR
Total RNA from HPAECs treated with NTCsi or BMPR-II siRNA was prepared using the QIAGEN RNeasy minikit, according to the manufacturer's instructions (QIAGEN). RNA was DNase treated, and 1 μg of total RNA reverse transcribed using a cDNA reverse-transcriptase kit (Applied Biosystems), according to the manufacturer's instructions. Real-time quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) was performed on a GeneAmp 7900HT. Expression of BMPR-II was determined using assay-on-demand primer sets (Applied Biosystems). Reactions were performed using an Applied Biosystems ABI7900. All data were analyzed using ABI7900 SDS Version 2.3 software (Applied Biosystems). Triplicate samples were run, transcripts were measured in picograms, and expression values were standardized to values obtained with control GAPDH (GAPDH Endogenous Control VIC/MGB Probe; Applied Biosystems).
Transmigration of neutrophils through cytokine-stimulated pulmonary artery endothelial cells under static conditions
Neutrophil transmigration was assessed using 6-well format Transwell filters in matching plates as previously described. Briefly, 1.5-mL neutrophil samples suspended in PBSA at a concentration of 1 × 106cells/mL were added to the upper chamber and allowed to transmigrate for 2 hours in an incubator maintained at 37°C, in 5% CO2. The number of transmigrated neutrophils was determined by counting the number of neutrophils in the lower chamber using a Vicell Series cell-viability analyzer (Beckman Coulter). The total percentage of transmigrated neutrophils was calculated by dividing the number of transmigrated neutrophils by the known number of neutrophils added.
Measurement of cytokines in HPAEC supernatants
The concentration of the inflammatory cytokines, IL-8 and IL-6 (BD OptEIA; BD Biosciences), epithelial neutrophil activating peptide-78 (ENA-78), and monocyte chemoattractant protein-1 (MCP-1; Quantikine; R&D Systems) were determined by commercially available sandwich enzyme-linked immunosorbent assay (ELISA), according to manufacturers instructions.
Assessment of HPAEC permeability by FITC albumin leakage
HPAECs were seeded onto 24-well inserts and cultured untreated or treated with siRNA-targeting BMPR-II or NTC siRNA as described earlier in “Methods.” Forty-eight hours after transfection, the HPAEC monolayer was washed twice with complete EGM, 200 μL of 1% (wt/vol) fluorescein isothiocyanate (FITC)–labeled albumin suspended in complete EGM was added to the upper chamber, and 800 μL of complete EGM containing 1% (wt/vol) BSA was added to the lower chamber. Leakage of FITC-labeled albumin into the lower chamber was assessed by removing 20 μL of sample from the lower chamber after 0.5, 1, and 2 hours, and fluorescence was measured using Envision (PerkinElmer).
Observation of leukocyte–endothelial cell interactions under conditions of flow
Leukocyte-endothelial interactions were observed using transmitted light microscopy on an inverted microscope (Olympus IX71). HPAECs were unstimulated or cytokine-stimulated in 6-well plates, then transferred into Ibidi slides at a concentration that yielded confluence within ∼ 2 hours. Slides were enclosed in a Perspex chamber at 37°C and attached to a perfusion system, which was set to provide a flow rate equivalent to a wall shear stress of 15 dyn/cm2. Physiologic shear stress in the arterial network ranges from 10 to 70 dyn/cm2.16 However, shear stress of 15-20 dyn/cm2 has been reported to be physiologic in normotensive patients.17
HPAECs were perfused with PBSA for 2 minutes to remove any excess TNF-α or TGF-β1. Neutrophils or mononuclear cells (1 × 106/mL) either untreated, pretreated for 10 minutes with the CXCR2 antagonist, SB265610 (Tocris Bioscience), or vehicle (dimethyl sulfoxide [DMSO]) in PBSA; 0.1%, vol/vol), were perfused over the endothelium for 2 minutes, followed by 2 minutes of cell-free PBSA. Video recordings of leukocyte-endothelial interactions were digitized and analyzed off-line using Image-Pro Plus Version 6.3 software (DataCell) as previously described.18 Leukocytes were determined as either (1) rolling, (2) firmly adherent to the endothelium, or (3) transmigrated through the endothelium. The number of leukocytes at each stage of recruitment (ie, rolling, firmly adherent, or transmigrated) was averaged per field and expressed per unit area and for the number of leukocytes perfused (mm2/106 cells perfused).
Animals
All animal procedures were conducted in accordance with the British Home Office regulations (Scientific Procedures) Act of 1986, United Kingdom. All studies were performed under a Home Office–approved project license.
L1cre(+);BMPR2f/f and L1cre(−);BMPR2f/f mice were obtained as a gift from Dr S. Paul Oh (University of Florida) and bred in-house. L1cre(+);BMPR2f/f and L1cre(−);BMPR2f/f mice were generated as previously described.10 Briefly, BMPR2f/f mice19 were intercrossed with L1cre20 and R26R mice (The Jackson Laboratory). L1cre(+)BMPR2+/f;R26R females were then intercrossed with the L1cre(+)BMPR2+/f;R26R males to produce L1cre(−)BMPR2f/f, L1cre(+)BMPR2f/f and L1cre(+)BMPR2+/f mice.
Mice were genotyped using PCR primer sets detecting the L1cre gene.
Measurement of pulmonary vascular leakage in vivo
Leakage of protein from the pulmonary vasculature of L1cre(−)BMPR2f/f or L1cre(+)BMPR2f/f mice was assessed by measuring the accumulation of Evans blue dye in the right lung.
Vehicle or Sugen (SU5416; 20 mg/kg) was administered to animals subcutaneously. Twenty-four hours later, Evans blue dye (30 mg/kg; Sigma-Aldrich) in sterile saline was injected via the tail vein. After 1 hour, the lungs were flushed with PBS, removed, and Evans Blue was extracted and quantitated in a spectrophotometer by measuring absorbance at 620 nm as previously described.21 Evans blue dye extravasation was expressed as ng Evans blue/mg dry tissue.
Measurement of MPO in vivo
The concentration of myeloperoxidase (MPO) in the right lung of L1cre(−)BMPR2f/f and L1cre(+)BMPR2f/f mice was measured using a murine MPO ELISA kit (HK210; Hycult Biotech). Lung homogenates previously assessed for vascular leakage using Evans blue (described above) were processed according to the manufacturer's instructions (Cambridge Bioscience), and the concentration of MPO was assessed using Envision (PerkinElmer).
Statistical Analysis
Data shown are the mean plus or minus SEM of a minimum of 3 independent experiments using a different HPAEC donor and different leukocyte donors. Statistical significance was achieved by comparisons of individual treatments by Student t test or by 2-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons test. P < .05 was considered significant.
Results
Expression of BMPR-II in HPAECs transfected with siRNA-targeting BMPR-II
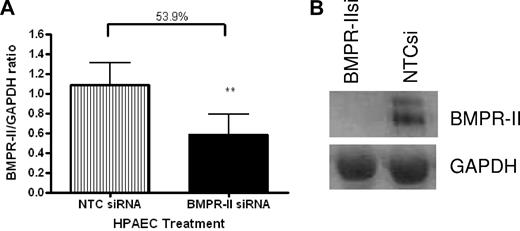
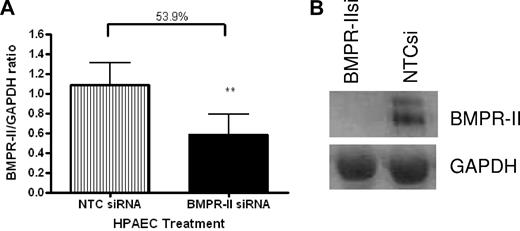
To confirm that BMPR-II expression in HPAECs was reduced as a result of transfection with siRNA-targeting BMPR-II, RNA was harvested from HPAECs transfected with siRNA-targeting BMPR-II or NTCsi for 48 hours and BMPR-II expression assessed using qPCR (Figure 1A) and Western blotting (Figure 1B). Figure 1A shows that transfection of HPAECs with siRNA-targeting BMPR-II resulted in approximately 50% loss of BMPR-II expression, compared with those HPAECs transfected with NTCsi. The reduction in mRNA expression for BMPR-II after siRNA treatment also resulted in a concomitant reduction in BMPR-II protein expression (Figure 1B).
BMPR-II expression in HPAECs treated with siRNA-targeting BMPR-II or a nontargeting control siRNA. RNA was harvested from mock-transfected HPAECs or those transfected with BMPR-IIsi or NTC siRNA. Expression of BMPR-II was determined using qPCR (TaqMan; A) using primers specific for BMPR-II or Western blotting (B). For TaqMan, data are the mean ± SEM for 5 independent experiments. **P < .001, compared with HPAECs transfected with NTCsi by Student t test.
BMPR-II expression in HPAECs treated with siRNA-targeting BMPR-II or a nontargeting control siRNA. RNA was harvested from mock-transfected HPAECs or those transfected with BMPR-IIsi or NTC siRNA. Expression of BMPR-II was determined using qPCR (TaqMan; A) using primers specific for BMPR-II or Western blotting (B). For TaqMan, data are the mean ± SEM for 5 independent experiments. **P < .001, compared with HPAECs transfected with NTCsi by Student t test.
BMPR-II is required for pulmonary endothelial cell barrier function
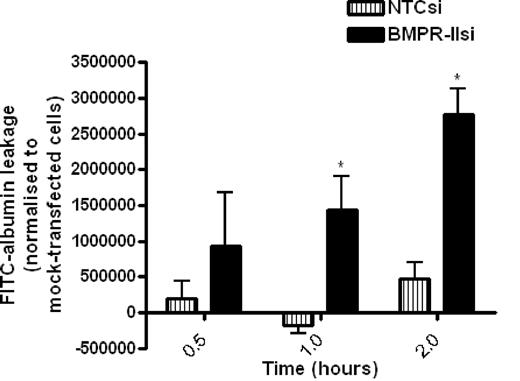
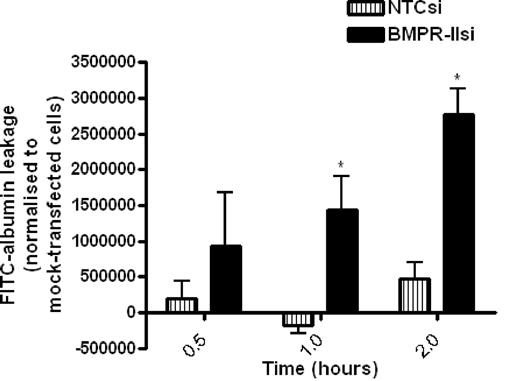
To examine the role of BMPR-II in HPAECs on barrier function, we assessed the kinetics of leakage of FITC-labeled albumin through mock-transfected HPAEC monolayers or HPAECs transfected with siRNA-targeting BMPR-II or NTC siRNA over time (Figure 2). The amount of FITC-albumin leakage through HPAEC monolayers with reduced BMPR-II expression was significantly higher than mock- and NTCsi-transfected controls after 1-hour incubation, and the leakage increased in a time-dependent manner.
Assessment of FITC-labeled albumin leakage across HPAEC monolayers transfected with siRNA-targeting BMPR-II, nontargeting control siRNA, or mock-transfected HPAEC. Mock-transfected HPAECs or HPAECs transfected with BMPR-IIsi or NTC siRNA were seeded onto Transwell filters. FITC-labeled albumin was added to the upper chamber, and leakage into the lower chamber was measured after 0.5, 1, and 2 hours and normalized to FITC-albumin leakage through mock-transfected HPAECs. Data are the mean ± SEM for 4 independent experiments. *P < .05, compared with NTCsi-transfected HPAECs by Student t test.
Assessment of FITC-labeled albumin leakage across HPAEC monolayers transfected with siRNA-targeting BMPR-II, nontargeting control siRNA, or mock-transfected HPAEC. Mock-transfected HPAECs or HPAECs transfected with BMPR-IIsi or NTC siRNA were seeded onto Transwell filters. FITC-labeled albumin was added to the upper chamber, and leakage into the lower chamber was measured after 0.5, 1, and 2 hours and normalized to FITC-albumin leakage through mock-transfected HPAECs. Data are the mean ± SEM for 4 independent experiments. *P < .05, compared with NTCsi-transfected HPAECs by Student t test.
Loss of BMPR-II in HPAECs leads to enhanced leukocyte transmigration under static and flow conditions
Having demonstrated that a reduction of BMPR-II on HPAECs compromises integrity of the endothelium, resulting in a more “leaky” monolayer, we sought to investigate these findings at a more functional level in terms of leukocyte recruitment.
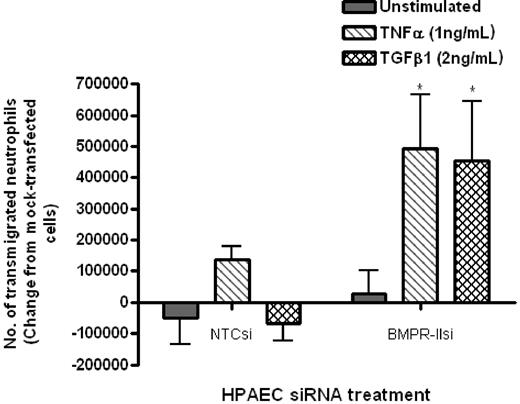
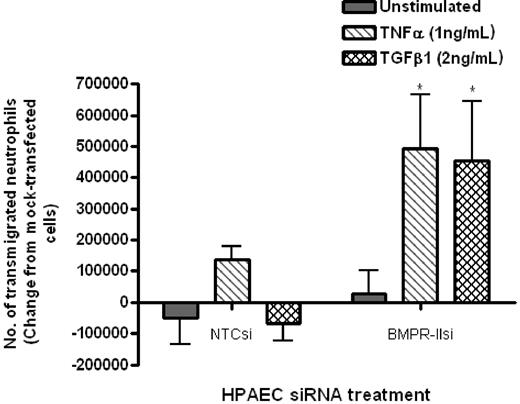
Initial experiments using a static transwell assay showed that neutrophil transmigration through HPAECs with reduced BMPR-II expression was significantly increased, compared with HPAECs with normal BMPR-II expression (NTCsi; Figure 3). This effect was only observed when endothelial monolayers were treated with TNF-α or TGF-β1.
Effect of reduced BMPR-II expression in HPAECs on leukocyte recruitment under static conditions. Mock-transfected HPAECs or HPAECs transfected with siRNA-targeting BMPR-II or nontargeting control siRNA were seeded onto Transwell filters, then unstimulated or stimulated with TNF-α for 4 hours or TGF-β1 for 24 hours. Neutrophil transmigration was assessed after 2 hours and shown as the change in the number of transmigrated neutrophils from mock-transfected HPAECs. Data are the mean ± SEM for 4 independent experiments. *P < .05, compared with NTCsi-transfected HPAECs by paired t test.
Effect of reduced BMPR-II expression in HPAECs on leukocyte recruitment under static conditions. Mock-transfected HPAECs or HPAECs transfected with siRNA-targeting BMPR-II or nontargeting control siRNA were seeded onto Transwell filters, then unstimulated or stimulated with TNF-α for 4 hours or TGF-β1 for 24 hours. Neutrophil transmigration was assessed after 2 hours and shown as the change in the number of transmigrated neutrophils from mock-transfected HPAECs. Data are the mean ± SEM for 4 independent experiments. *P < .05, compared with NTCsi-transfected HPAECs by paired t test.
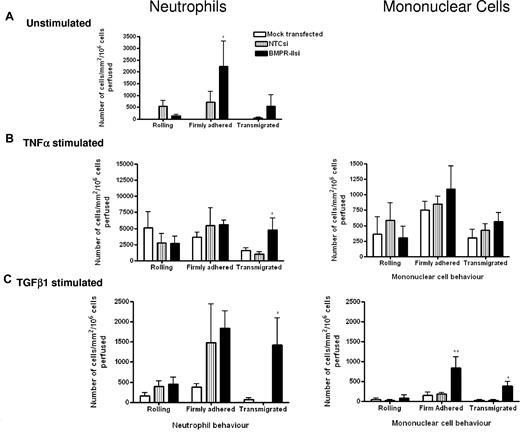
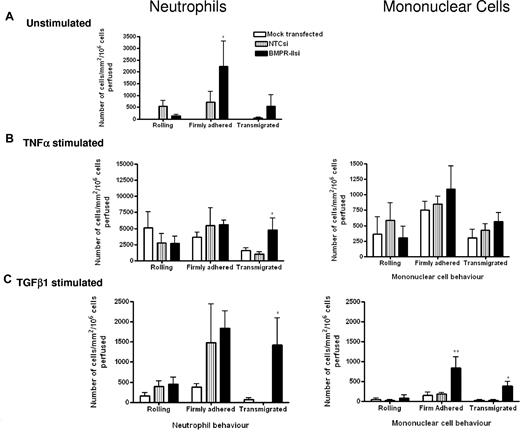
To define the stage at which leukocyte recruitment was enhanced by the loss of BMPR-II, we extended our studies to a flow-based model, which allowed direct observation of leukocyte-endothelial interactions in real time. When the endothelium was unstimulated (Figure 4A), the number of neutrophils captured from flow was similar for HPAECs with reduced BMPR-II expression and NTC siRNA-transfected controls. However, the number of neutrophils that had firmly adhered to the endothelium was significantly increased in those HPAECs with reduced BMPR-II expression, compared with NTC siRNA-treated controls (Figure 4A). With regard to transmigration, little transmigration was observed through HPAECs treated with NTC siRNA; however, some transmigration was supported in those HPAECs with reduced BMPR-II expression, although this did not reach statistical significance (Figure 4A). Upon stimulation with TNF-α, HPAECs supported neutrophil adhesion and transmigration (Figure 4B). The number of neutrophils captured from flow and those firmly adhered to the endothelium was similar, regardless of BMPR-II expression (Figure 4B). However, there was more efficient transmigration through the endothelium with reduced BMPR-II expression, compared with cells treated with NTCsi.
Effect of reduced BMPR-II expression in HPAECs on leukocyte recruitment under physiologic flow conditions. Mock-transfected HPAECs or HPAECs transfected with siRNA targeting BMPR-II or nontargeting control siRNA were seeded into Ibidi IV flow slides, then unstimulated (A) or stimulated with TNF-α for 4 hours (B) or TGF-β1 for 24 hours (C). Neutrophils (left panel) or mononuclear cells (right panel) were flowed across the endothelial monolayer for 2 minutes, followed by washout. Adherent leukocytes were deemed as rolling, firmly adherent, or transmigrated. Time zero was the start of leukocyte perfusion. Data are the mean ± SEM for 5 independent experiments for TNF-α–stimulated HPAECs and 6 experiments for unstimulated and TGF-β1–stimulated HPAECs. *P < .05, **P < .01, compared with NTCsi-transfected HPAECs by Student t test.
Effect of reduced BMPR-II expression in HPAECs on leukocyte recruitment under physiologic flow conditions. Mock-transfected HPAECs or HPAECs transfected with siRNA targeting BMPR-II or nontargeting control siRNA were seeded into Ibidi IV flow slides, then unstimulated (A) or stimulated with TNF-α for 4 hours (B) or TGF-β1 for 24 hours (C). Neutrophils (left panel) or mononuclear cells (right panel) were flowed across the endothelial monolayer for 2 minutes, followed by washout. Adherent leukocytes were deemed as rolling, firmly adherent, or transmigrated. Time zero was the start of leukocyte perfusion. Data are the mean ± SEM for 5 independent experiments for TNF-α–stimulated HPAECs and 6 experiments for unstimulated and TGF-β1–stimulated HPAECs. *P < .05, **P < .01, compared with NTCsi-transfected HPAECs by Student t test.
When the endothelium was stimulated with TGF-β1, neutrophil adhesion and transmigration through mock-transfected cells was not supported (Figure 4C). The number of neutrophils captured from flow and those firmly adhered to the endothelium was similar in those HPAECs with reduced BMPR-II expression and NTC siRNA-transfected controls. However, transmigration was only significantly enhanced through the endothelium with reduced BMPR-II expression.
Because the majority of inflammatory cells found around the plexiform lesions of PAH patients include lymphocytes and monocytes,6 we repeated the flow-based experiments using mononuclear cells to investigate that the same phenomenon observed with neutrophils was true of monocytes and lymphocytes. Unstimulated HPAECs did not support mononuclear cell recruitment; however, stimulation with TNF-α supported adhesion and transmigration, with no observed difference between HPAECs with normal or reduced BMPR-II expression levels (Figure 4B). Upon TGF-β1 stimulation, mononuclear cell adhesion to HPAEC, with normal BMPR-II expression was minimal, whereas there was a 5-fold increase in the number of mononuclear cells firmly adherent to HPAECs with reduced BMPR-II expression (Figure 4C). Similarly, significantly enhanced transmigration was observed in the context of reduced BMPR-II expression (Figure 4C).
Effect of reduced BMPR-II expression on cell-surface expression of adhesion molecules
To investigate the mechanisms by which loss of BMPR-II expression in HPAECs may be supporting more efficient leukocyte transmigration, we examined the surface expression of the capture receptors, P- and E-selectin, and the adhesion molecules, vascular cell adhesion molecule (VCAM) and ICAM-1 (supplemental Figure 1; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Surprisingly, there was no difference in the surface expression of any of these molecules between HPAECs, which had reduced BMPR-II expression, and those with normal BMPR-II expression.
Effect of reduced BMPR-II expression in HPAECs on release of inflammatory cytokines
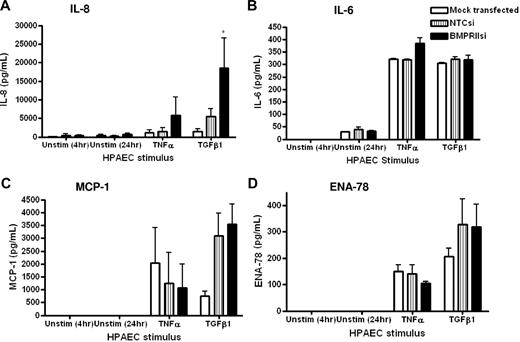
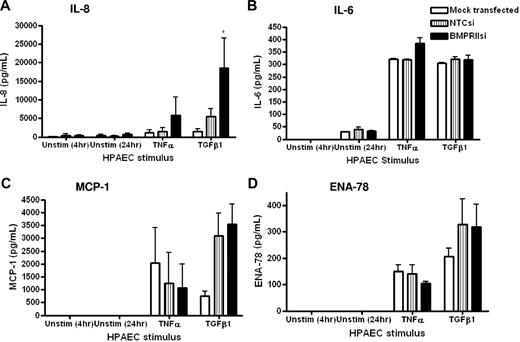
Because we found that the loss of endothelial BMPR-II resulted in no change in the surface expression of adhesion molecules, we examined the possibility that loss of endothelial BMPR-II may promote leukocyte transmigration by altering the release of inflammatory cytokines. Thus, we examined the secretion of IL-8 (Figure 5A), IL-6 (Figure 5B), monocyte chemoattractant protein-1 (MCP-1; Figure 5C), and epithelial neutrophil-activating peptide 78 (ENA-78; Figure 5D) from HPAECs to assess the impact of the reduction of BMPR-II expression on the levels of cytokines released. Unstimulated levels of all cytokines tested were below the level of detection. In contrast, stimulation of HPAECs with TNF-α or TGF-β1 increased the release of all cytokines tested. Those endothelial monolayers with reduced BMPR-II expression showed a significant increase in the release of certain inflammatory cytokines, compared with HPAECs transfected with NTCsi, which also appeared to be stimulus specific. For example, stimulation of the endothelium with TNF-α resulted in a modest increase in the release of IL-8 and IL-6 from HPAECs with reduced BMPR-II expression, compared with NTCsi RNA controls (Figure 5A-B). In contrast, stimulation with TGF-β1 resulted in a significant increase in the release of IL-8, compared with NTCsi RNA controls (Figure 5A).
Effect of reduced HPAEC BMPR-II expression on secretion of IL-8, IL-6, MCP-1, and ENA-78. Concentrations of IL-8 (A), IL-6, (B), MCP-1 (C), and ENA-78 (D) in supernatants from mock-transfected HPAECs or HPAECs transfected with siRNA-targeting BMPR-II or nontargeting control siRNA were determined by sandwich ELISA. Data are the mean ± SEM for a minimum of 3 independent experiments. *P < .05, compared with NTCsi-transfected cells by Student t test.
Effect of reduced HPAEC BMPR-II expression on secretion of IL-8, IL-6, MCP-1, and ENA-78. Concentrations of IL-8 (A), IL-6, (B), MCP-1 (C), and ENA-78 (D) in supernatants from mock-transfected HPAECs or HPAECs transfected with siRNA-targeting BMPR-II or nontargeting control siRNA were determined by sandwich ELISA. Data are the mean ± SEM for a minimum of 3 independent experiments. *P < .05, compared with NTCsi-transfected cells by Student t test.
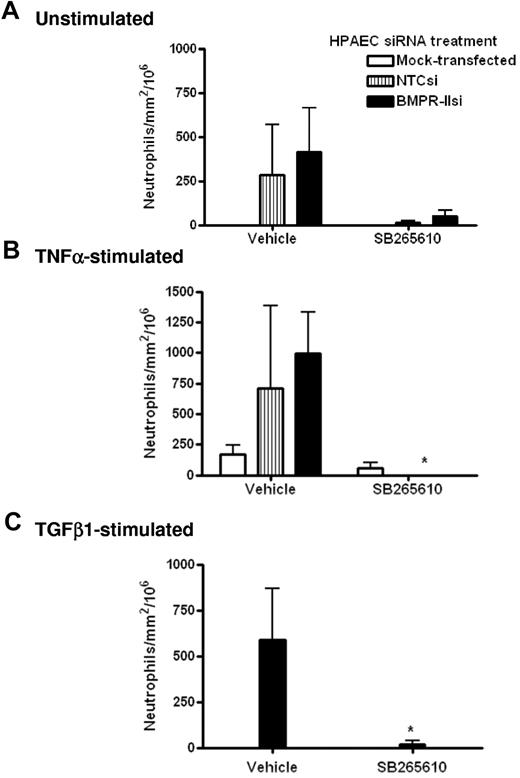
Effect of the CXCR2 antagonist, SB265610, on neutrophil transmigration through HPAECs with reduced BMPR-II expression
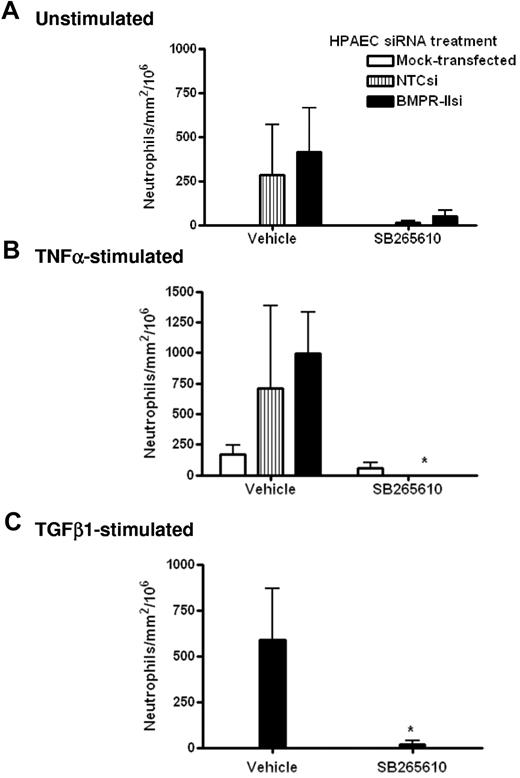
To further elucidate the molecular mechanism by which transmigration was facilitated by the reduction of endothelial BMPR-II expression, we investigated the effects of the CXCR2 antagonist, SB265610, on neutrophil transmigration through HPAECs transfected with BMPR-II siRNA. In unstimulated endothelial monolayers (Figure 6A), treatment with SB265610 tended to reduce the number of neutrophil transmigrating through endothelial monolayers with normal (NTCsi) and reduced BMPR-II expression (BMPR-IIsi); however, this was not statistically significant.
Effect of CXCR2 antagonist SB265610 on neutrophil transmigration through HPAEC with reduced BMPR-II expression. Mock-transfected HPAECs or HPAECs transfected with siRNA-targeting BMPR-II or nontargeting control siRNA were seeded into Ibidi IV flow slides, then unstimulated (A), stimulated with TNF-α for 4 hours (B), or TGF-β1 for 24 hours (C). Neutrophils were incubated with the CXCR2 antagonist, SB265610, or vehicle at 37°C for 10 minutes before perfusion across the endothelial monolayer for 2 minutes, followed by washout. Data are the mean ± SEM for 6 independent experiments for unstimulated HPAECs and 3 experiments for TNF-α and TGF-β1–stimulated HPAECs. *P < .05, compared with cells treated with vehicle by Student t test.
Effect of CXCR2 antagonist SB265610 on neutrophil transmigration through HPAEC with reduced BMPR-II expression. Mock-transfected HPAECs or HPAECs transfected with siRNA-targeting BMPR-II or nontargeting control siRNA were seeded into Ibidi IV flow slides, then unstimulated (A), stimulated with TNF-α for 4 hours (B), or TGF-β1 for 24 hours (C). Neutrophils were incubated with the CXCR2 antagonist, SB265610, or vehicle at 37°C for 10 minutes before perfusion across the endothelial monolayer for 2 minutes, followed by washout. Data are the mean ± SEM for 6 independent experiments for unstimulated HPAECs and 3 experiments for TNF-α and TGF-β1–stimulated HPAECs. *P < .05, compared with cells treated with vehicle by Student t test.
When the endothelium was stimulated with TNF-α or TGF-β1, treatment with SB265610 significantly ablated the number of transmigrated neutrophils (Figure 6B-C). Interestingly, it was observed that treatment with SB265610 tended to shift the number of neutrophils that transmigrate to those that are rolling, with little effect on neutrophils undergoing firm adhesion (data not shown). This was observed in unstimulated, TNF-α, and TGF-β1–stimulated HPAECs, irrespective of siRNA treatment.
BMPR-II is required for pulmonary endothelial cell barrier function in vivo
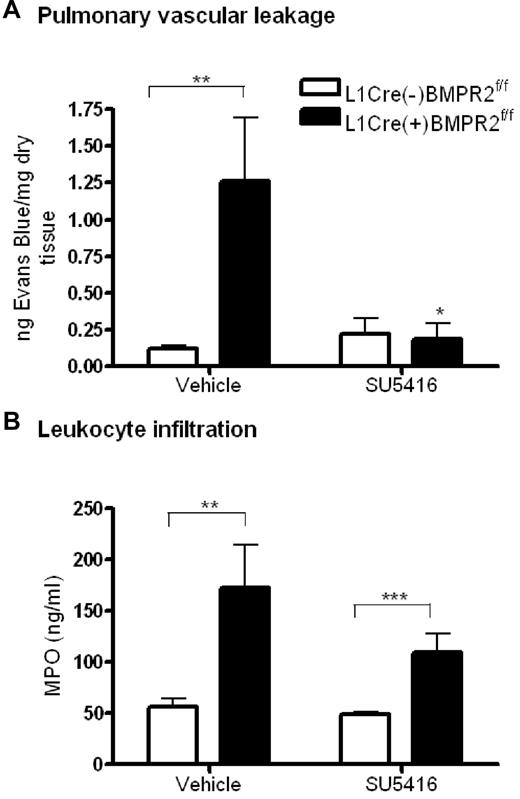
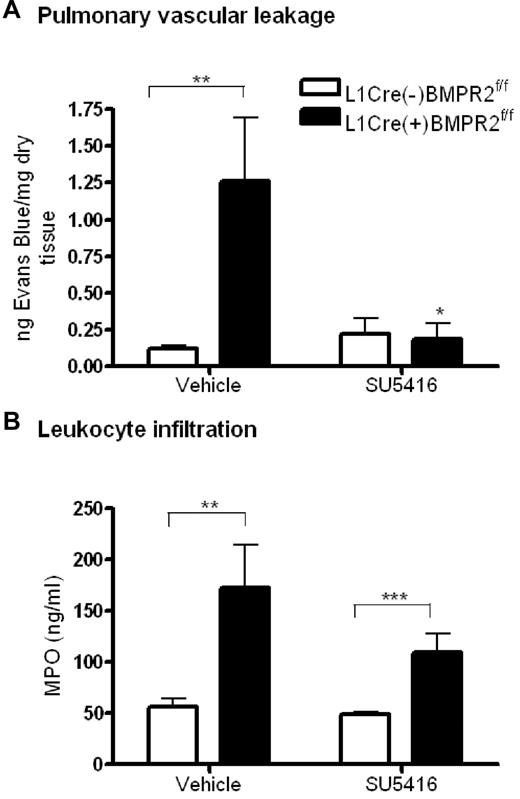
To determine whether the loss of barrier function with reduced BMPR-II expression seen in vitro was translated in vivo, we assessed the leakage of Evans Blue from the pulmonary vasculature in L1cre(+)BMPR2f/f mice (which spontaneously develop PAH).10 Pulmonary vascular leakage was significantly increased in L1cre(+)BMPR2f/f mice, compared with L1cre(−)BMPR2f/f (Figure 7A). However, there was no difference in vascular leakage between either genotype of mice upon treatment with the vascular endothelial growth factor (VEGF) receptor inhibitor, SU5416 (Figure 7A).
Assessment of vascular leakage and leukocyte infiltration in endothelial-restricted BMPR-II–deficient mice. (A) To assess vascular leakage, L1cre(+)BMPR2f/f mice or L1cre(−)BMPR2f/f mice were injected with SU5416 or vehicle subcutaneously for 23 hours. Evans Blue dye was then administered intravenously via the tail vein for 1 hour, after which the lungs were flushed with PBS and the heart/lung block excised. Evans blue was extracted from the right lung, quantified, and expressed as nanograms of Evans Blue per milligram of dry lung tissue. Data are the mean ± SEM for 7 L1cre(+)BMPR2f/f and 9 L1cre(−)BMPR2f/f mice. In panel A, ANOVA showed a significant effect of treatment with SU5416 and genotype P < .05. *P < .05, **P < .01, compared with L1cre(−)BMPR2f/f animals. (B) To assess leukocyte infiltration, the concentration of MPO in the right lung of L1cre(+)BMPR2f/f and L1cre(−)BMPR2f/f mice was determined. In panel B, ANOVA showed a significant effect of genotype P < .001. **P < .01, ***P < .001, compared with L1cre(−)BMPR2f/f animals.
Assessment of vascular leakage and leukocyte infiltration in endothelial-restricted BMPR-II–deficient mice. (A) To assess vascular leakage, L1cre(+)BMPR2f/f mice or L1cre(−)BMPR2f/f mice were injected with SU5416 or vehicle subcutaneously for 23 hours. Evans Blue dye was then administered intravenously via the tail vein for 1 hour, after which the lungs were flushed with PBS and the heart/lung block excised. Evans blue was extracted from the right lung, quantified, and expressed as nanograms of Evans Blue per milligram of dry lung tissue. Data are the mean ± SEM for 7 L1cre(+)BMPR2f/f and 9 L1cre(−)BMPR2f/f mice. In panel A, ANOVA showed a significant effect of treatment with SU5416 and genotype P < .05. *P < .05, **P < .01, compared with L1cre(−)BMPR2f/f animals. (B) To assess leukocyte infiltration, the concentration of MPO in the right lung of L1cre(+)BMPR2f/f and L1cre(−)BMPR2f/f mice was determined. In panel B, ANOVA showed a significant effect of genotype P < .001. **P < .01, ***P < .001, compared with L1cre(−)BMPR2f/f animals.
Having demonstrated that loss of BMPR-II in cultured HPAEC results in increased leukocyte transmigration, we sought to investigate if leukocyte infiltration was also increased in the lungs of the L1cre(+)BMPR2f/f mice. Using an assay to measure MPO activity in lung homogenates as a measure of leukocyte infiltration,22 we found that leukocyte infiltration into the lungs of L1cre(+)BMPR2f/f mice was significantly increased, compared with L1cre(−)BMPR2f/f mice (Figure 7B). This was observed in both vehicle- and SU5416-treated mice, although infiltration into the lungs of vehicle-treated mice was more marked than those that were treated with SU5416.
Discussion
Mutations in the BMPR-II gene that leads to reduced expression of functional BMPR-II have been implicated in the pathogenesis of PAH. However, less than half of carriers develop PAH, suggesting that additional factors may be required to “trigger” the onset of the disease in predisposed individuals. This theory is supported in genetic mouse models, whereby heterozygous BMPR-II-null mice only develop PAH when an additional inflammatory stimulus is applied.9 Despite this, the role of BMPR-II in regulating the response of PAECs to inflammatory insult has not been clearly defined.
It is now recognized that endothelial dysfunction is likely to play a key role in the initiation and progression of PAH.23 Surprisingly, the role of BMPR-II in regulating the normal barrier function of PAEC has been poorly defined. In this study, we show that BMPR-II plays a significant role in regulating the barrier function of PAECs in vitro and in vivo, evidenced by increased permeability of the monolayer and increased leukocyte recruitment observed in cells with reduced BMPR-II expression.
Our results imply that BMPR-II may act to dampen inflammatory signals in the pulmonary endothelium, and that reduced BMPR-II expression and the resultant reduction in the natural barrier function of the endothelium may predispose individuals to inflammation-induced pulmonary vascular damage.
A major role of the vascular endothelium is to provide a barrier between blood and tissues, thus preventing underlying stromal cells from exposure to growth factors present in the blood. In PAH, it has been suggested that a loss of endothelial-barrier integrity may result in the abnormal exposure of the underlying smooth muscle cells to growth factors, leading to their uncontrolled proliferation. Many investigators have suggested that abnormal BMP signaling is likely to lead to an alteration in the permeability of the endothelial cells within the vascular wall.23-25 However, until now, there was no experimental evidence to support this hypothesis. In this study, we found that integrity of the endothelial monolayer was compromised when BMPR-II expression was reduced, resulting in the development of a more “leaky” endothelial monolayer. In support of this, we also show that vascular permeability is increased in L1cre(+)BMPR2f/f mice. Interestingly, the use of SU5416 (a reported inhibitor of VEGFR2)26 reduced the increased vascular permeability observed in the L1cre(+)BMPR2f/f mice. VEGF is known to be a potent inducer of vascular permeability in the lung.27 Serum levels of VEGF have been shown to be elevated in PAH patients28-30 and critical in the formation of plexiform lesions.28,29 Therefore, it is possible that VEGF-VEGFR2 interactions in our mouse model may enhance vascular permeability, possibly through the regulation of gap junctional proteins, and the blockade of VEGR2 with SU5416 prevented reorganization of cell junctions and therefore retained vascular permeability.
In light of the fact that SU5416 has been demonstrated to exacerbate PAH in rodent hypoxia models of PAH,31 it will be of interest to determine the effect of chronic application of SU5146 on the development of PAH in the L1cre(+)BMPR2f/f model. Our data suggest that SU5416 may be therapeutically beneficial by preventing the recruitment of leukocytes in to the pulmonary vascular wall in the L1cre(+)BMPR2f/f model; however, this early beneficial effect of VEGFR2 inhibition may be overridden by abrogation of other protective VEGFR2-related mechanisms, such as neoangiogenesis in the pulmonary vasculature32 and adaptive right ventricular hypertrophy.33
Our finding that BMPR-II functionally regulates endothelial-barrier property is consistent with prior observations in the pulmonary hypertensive vasculature. Wojciak-Stothard et al found that the pulmonary endothelium from pulmonary hypertensive piglets had a stable abnormal phenotype, with increased Rac1 and RhoA activity, actin stress fiber formation, and increased vascular permeability.34 Abnormalities in RhoA activity and actin stress-fiber formation have been implicated in a variety of other preclinical models of PAH.35 While increased endothelial permeability may contribute to the ability of immune, progenitor, and inflammatory cells to enter the pulmonary artery wall, it is unlikely to contribute to the development of pulmonary edema, per se. Townsley et al36 demonstrated that a thickened endothelial basement membrane, as is seen in humans with PAH, counterbalanced hydrostatic driving forces in chronically paced dogs, resulting in decreased filtration coefficient and no change in edema formation. In addition, human PAH is characterized by relatively homogenous precapillary flow restriction, which further decreases the driving pressure for fluid extravasation into the lung.
It has been demonstrated that an increase in vascular permeability is linked to an increase in leukocyte tissue retention.37,38 With regard to PAH, the identification of leukocytes, such as monocytes/macrophages and lymphocytes, in plexiform lesions of pulmonary hypertensive vessels39 supports the theory that inflammatory cells play a role in the initiation and progression of PAH. It has also been suggested that loss of BMPR-II may result in increased permeability to inflammatory cells.24 In support of this, we observed increased leukocyte transmigration through PAEC with reduced BMPR-II expression and increased leukocyte infiltration into the lung of L1cre(+)BMPR2f/f mice. We have not assessed which population of leukocytes are extravasating into the pulmonary artery wall of the L1cre(+)BMPR2f/f mice; however, previous histologic work with this mouse model has demonstrated that CD68+ mononuclear cells are the predominant inflammatory cell type present.10
To dissect the stage at which leukocyte infiltration was facilitated by loss of endothelial BMPR-II expression, we used a flow-based model, whereby each stage of leukocyte recruitment could be observed in real time. Here, loss of BMPR-II expression in HPAECs markedly increased leukocyte transmigration, with little effect on the early stages of leukocyte recruitment (ie, rolling and firm adhesion). Although this was evident, to some extent, in “resting” endothelium, transmigration was dramatically increased upon HPAEC stimulation with TNF-α and TGF-β1.
To try and elucidate the molecular mechanisms mediating this observation, initial investigations showed that loss of BMPR-II did not alter the expression of the capture receptors, P- and E-selectin, or the adhesion molecules, ICAM-1 and VCAM-1. However, we sought to investigate the possibility that BMPR-II may regulate the secretion of proinflammatory cytokines, thus influencing leukocyte transmigration. Elevated circulating levels of proinflammatory cytokines in patients with PAH has been widely reported40,41 ; however, it has not been shown that release of these cytokines originates from the endothelium or that BMPR-II may play a role in regulating their secretion. In this study, we found increased secretion of the proinflammatory cytokine, IL-8, from HPAECs with reduced BMPR-II expression when stimulated with TGF-β1 and a modest increase in the secretion of IL-8 upon TNF-α stimulation. Because IL-8 can act through CXCR2,42 we hypothesized that the increased transmigration observed through HPAECs with reduced BMPR-II may have been acting through CXCR2. Our results showed that transmigration could be abolished by the inhibition of CXCR2, confirming our hypothesis that the increased transmigration observed through HPAECs with reduced BMPR-II expression was CXCR2 mediated in this model. These findings strongly suggest that BMPR-II can regulate the secretion of proinflammatory cytokines, and that a reduction in BMPR-II expression may result in the endothelium becoming susceptible to an inappropriate release of proinflammatory cytokines upon inflammatory insult.
We have not determined whether the increased leukocyte lung-tissue levels observed in L1cre(+)BMPR2f/f mice also occurs via an active process. Hagen and colleagues previously demonstrated that smooth muscle–restricted expression of dominant-negative BMPR2 (BMPR2del4x) resulted in elevated peripheral blood levels of proinflammatory cytokines.8 If a similar increase in circulating proinflammatory cytokines occurs in the L1cre(+)BMPR2f/f mouse model, then the observed leukocyte extravasation may also be occurring in vivo by an active process. Experiments are in progress to assess if this is, indeed, the case.
In summary, the findings of our studies indicate that BMPR-II plays a role in maintaining vascular integrity and dampening inflammatory signals in the pulmonary vasculature, and that loss of BMPR-II may predispose the pulmonary vasculature to heightened inflammatory responses upon inflammatory challenge. It will be of interest to assess if the expression of mutated forms of BMPR-II also reduces the pulmonary endothelial barrier function in a similar fashion to loss of receptor expression. In this regard, recently described data have shown that alterations in the actin organization–related pathways are evident in transgenic mouse models expressing BMPR-II R899X receptors from a universal promoter. Interestingly, the investigator speculates that the gene-expression changes observed may be expected to result from increased permeability of the pulmonary vascular wall to inflammatory cells.24 From these studies, it is tempting to speculate that pharmacologic enhancement of barrier function could slow or inhibit the initiation of PAH. Future studies are in progress to address this possibility.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.J.B. designed and performed the in vitro and in vivo experiments, analyzed the data, and contributed to the writing of the manuscript; L.I.C provided help performing the in vivo experiments; A.M.H. and C.W. provided intellectual input; D.B. provided project supervision; and D.B., A.M.H., and D.M.R. contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David C. Budd, Respiratory Disease Area, Novartis Institutes for BioMedical Research, Wimblehurst Rd, Horsham, West Sussex RH12 5AB, UK; e-mail: david.budd@novartis.com.