In this issue of Blood, Maloney et al report that the inferior outcome of ALL in children with DS is explained by the infrequency of the typical genetic subtypes characterizing common ALL.1
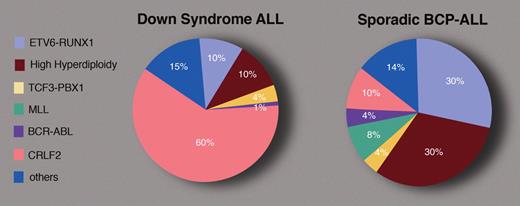
Approximate frequencies of some of the leukemia-defining cytogenetic lesions in BCP-ALL in children with and without DS. Professional illustration by Marie Dauenheimer.
Approximate frequencies of some of the leukemia-defining cytogenetic lesions in BCP-ALL in children with and without DS. Professional illustration by Marie Dauenheimer.
Children with Down syndrome (DS) have a markedly increased risk of both myeloid and lymphoblastic leukemias.2 Whereas the myeloid leukemias have unique clinical characteristics, the acute lymphoblastic leukemias (ALLs) of DS have a similar clinical appearance to the “common” B-cell precursor (BCP) ALLs seen in children without DS, with the notable absence of infant leukemias.3 The peak age is approximately 5 years. The immunophenotype is typical for BCP ALL, namely positive for CD10, CD19, and CD79a, and usually classified into the standard National Cancer Institute risk group. Yet, Maloney et al report that despite this phenotypic similarity, the spectrum of genetic anomalies in DS-ALL is markedly different from sporadic ALL (see figure).
The current study describes a systematic genotyping of all children with BCP-ALL, enrolled in prospective treatment protocols of the Children's Oncology Group (COG) between 1999 and 2005. Among 2811 children, 80 had DS. Maloney and colleagues also report the frequency of the ETV6-RUNX1 anomaly in an additional cohort of 118 DS-ALLs among 4365 children with BCP-ALL enrolled in COG studies since 2005. The results of this large prospective study are remarkably similar to the report by Forestier et al from the iBFM Study Group of 215 DS-ALLs compiled from multiple cytogenetics laboratories.4 Both suggest that the frequencies of high-hyperdiploidy and ETV6-RUNX1 translocations in DS-ALL are close to 10%. (The prevalence of a 2.5% ETV6-RUNX1 aberration mentioned in the abstract was amended to 11.5% in the follow-up study reported in the discussion section of the COG study.) Thus, only one-fifth of DS-ALLs carry the 2 most frequent genetic anomalies characterizing approximately 60% of sporadic childhood BCP-ALL.
So what are the common somatic genetic anomalies that cooperate with constitutional trisomy 21 (cT21) in DS-ALL? Recent research has identified genomic aberrations causing the expression of cytokine receptor CRLF2 in approximately 60% of DS-ALLs.5-7 In contrast, these lesions occur in 10% or fewer cases of sporadic childhood ALL.7-9 Although prospective analysis is still required, the expression of CRLF2 seems to be associated with poorer prognosis.5,8-10
How cT21 enhances the risk of ALL has remained a mystery and studies of sporadic ALL with excess chromosome 21 (eg, hyperdiploid ALL) have not contributed to solving that mystery. The same genes are overexpressed from the trisomic chromosome 21 in DS and in sporadic ALL,5 yet these leukemias are different. Perhaps the presence of cT21 at the earliest stages of embryogenesis impacts B-cell development and consequently the risk for leukemic transformation in DS. One possibility is that a prolonged arrest in early B-cell developmental stages in which the V(D)J recombination machinery is active might explain the chromosomal aberrations involving CRLF2 or other translocations to the IgH locus that are more frequent in DS-ALL. Consistent with this hypothesis is the aberrant expression of DNA damage genes in DS-ALL, suggesting the presence of lymphocytic-specific genomic instability.5 Alternatively, cT21 in the bone marrow microenvironment of DS may provide positive selection pressures for preleukemic cells carrying the cytokine receptor CRLF2.
Treatment of DS-ALLs is challenging. Fatal infections in children with DS treated with chemotherapy are believed to be responsible for the inferior outcome of DS-ALLs. Maloney et al dispute this common belief. They demonstrate that the major cause of the poorer outcome of DS-ALL is the lower prevalence of the good prognostic sentinel cytogenetic lesions, namely ETV6-RUNX1 fusion and trisomies 4 and 10. Their findings confirm a previous report from the Children's Cancer Group.11 Thus, the clinician faced with a patient with DS and ALL has difficult choices. Intensive chemotherapy is likely to cause life-endangering toxicity but is also required for cure, especially if the leukemia lacks the ETV6-RUNX1 translocation or hyperdiploidy. However, there may be a light at the end of this tricky maze. The activation of the CRLF2-JAK-STAT signaling pathway in the majority of DS-ALLs suggests a therapeutic potential for JAK inhibitors. If confirmed in clinical trials, this therapy will target the unique biological properties of ALLs in children with DS.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■