Abstract
Angiogenesis is controlled by signals that stimulate motility in endothelial cells at the tips of vascular sprouts while maintaining cell-cell adhesion in the stalks of angiogenic sprouts. We show here that Gs-linked G protein–coupled receptor activation of cAMP-dependent protein kinase (PKA) plays an important role in regulating the switch between endothelial cell adhesion and migration by activating C-terminal Src kinase, leading to inhibition of pp60Src. Activated PKA blocks pp60Src-dependent vascular endot helial-cadherin phosphorylation, thereby stimulating cell-cell adhesion while suppressing endothelial cell polarization, motility, angiogenesis, and vascular permeability. Similar to the actions of Notch and Dll4, PKA activation blocks sprouting in newly forming embryonic blood vessels, while PKA inhibition promotes excessive sprouting in these vessels. These findings demonstrate that G protein–coupled receptors and PKA regulate vascular sprouting during angiogenesis by controlling endothelial cell migration and cell-cell adhesion through their actions on pp60Src.
Introduction
During angiogenesis, endothelial cell sprouting from preexisting vessels leads to generation of new blood vessels. During this process, stably adherent “tip” endothelial cells loosen their cell-cell adhesive contacts and exhibit increased cell migration while stalk cells proliferate but continue to exhibit strong cell-cell adhesion.1,2 These events are controlled by vascular endothelial growth factor-A (VEGF-A) induced Dll4 expression in tip cells and by Notch expression in stalk cells. Notch signaling prevents induction of the tip cell migratory phenotype, as perturbation of Dll4 and Notch interactions leads to generation of more tip cells and excessive vascular sprouting.1,2
A rise in VEGF-A expression during angiogenesis promotes endothelial cell migration and inhibits vascular endothelial(VE)–cadherin–mediated endothelial cell-cell adhesion.3-6 The 150-amino acid VE-cadherin cytoplasmic tail binds to β-catenin; this complex then interacts with α-catenin, which facilitates association with the actin cytoskeleton and strengthens cell-cell adhesion.6 Loss of cadherin function and or expression is associated with increased cell migration, indicating that molecules that regulate cadherin function play key roles in the regulation of the switch from a stably adherent to a migratory cellular phenotype.6
VEGF-A activates pp60Src, promoting endothelial cell migration and inhibiting endothelial cell-cell adhesion. VEGF-A activation of pp60Src promotes cell migration by modulating focal adhesion turnover. VEGF-A also phosphorylates VE-cadherin, leading to β-catenin dissociation from VE-cadherin and inhibition of cell-cell adhesion.3-6 Src is a central regulator of endothelial cell migration and cell-cell adhesion and consequently, Src inhibitors block angiogenesis and vascular permeability.
Cellular migration plays a critical role during embryonic development and angiogenesis.7-9 Migration begins with formation of new integrin mediated cell-substrate adhesions at the leading edge of the cell and requires de-adhesion at the rear of the cell. Formation and dissolution of these cell-substrate adhesions, focal adhesions, plays a key role in cell motility and is regulated by pp60Src. Inhibition of pp60Src blocks focal adhesion turnover and inhibits cell migration.9
While many stimuli such as VEGF-A, basic fibroblast growth factor (bFGF), interleukin-8, and stromal cell–derived factor-1 promote endothelial cell migration and angiogenesis, other stimuli such as parathyroid hormone, parathyroid hormone-related protein, epinephrine, prostaglandin E2, retinoic acid, and CXCL10 (IP-10), suppress cell migration and/or inhibit angiogenesis in vivo.10-14 These ligands for Gs-linked G protein-coupled receptors (GPCRs), activate adenyl cyclase and, subsequently, cAMP-dependent protein kinase (PKA). Pharmacologic or genetic activation of PKA inhibits cell migration in endothelial and other cells.15-17 Importantly, while Gαs-linked GPCRs, such as the parathyroid hormone-related peptide/parathyroid hormone (PTHrP/PTH) receptor (PTHR1)10 and β-adrenergic receptor,11 activate PKA and inhibit angiogenesis, Gαi-linked GPCRs, such as CXCR4, the stromal cell–derived factor-1α receptor, and CXCR1, the interleukin-8 receptor, suppress PKA activation and promote endothelial cell motility and angiogenesis.18,19 Together, these findings indicate that PKA plays a central role in the regulation of endothelial cell migration and adhesion during vascular remodeling events.
Here we report that PKA activation simultaneously inhibits endothelial cell migration and stimulates cell-cell adhesion, thereby blocking angiogenesis and vascular leak. Activated PKA stimulates endothelial cell c-Src kinase (Csk), which inhibits Src activation by phosphorylating the terminal Src Y529. Inhibition of Src activity suppresses endothelial cell migration by inhibiting focal adhesion turnover and stimulating cell-cell adhesion. These studies indicate that PKA plays a key role in the regulation of vascular sprouting by stimulating endothelial cell adhesion and inhibiting cell migration and imply that it may act similarly to Notch/Dll4 to control endothelial cell fate.
Methods
Additional methods are in the supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All culture of zebrafish and their embryos were approved by the University of California, San Diego Institutional Animal Care and Use Committee.
PKA assays
Human umbilical vein endothelial cells (HUVECs) on vitronectin-coated plates were treated with medium or 20μM forskolin/500μM dibutyryl cAMP or vehicle control (0.4% dimethyl sulfoxide [DMSO]) for 0-60 minutes. Cells were extracted with 50mM Tris, pH 7.5, 10mM benzamidine, 5mM EDTA (ethylenediaminetetraacetic acid), 10mM EGTA (ethyleneglycoltetraacetic acid), 1mM phenylmethylsulfonyl fluoride, 50mM β-mercaptoethanol on ice. PKA activity was assessed using a nonradioactive kit (539484; Calbiochem).
Csk and Src kinase assays
Csk was immunoprecipitated with 3 μg anti-Csk antibody (C-20; Santa Cruz Biotechnology) from 300 μg RIPA lysates of cells treated with or without 30 μg/mL forskolin/250μM dibutyryl cAMP for 15 minutes at 37°C. Replicate immunoprecipitates were electrophoresed and immunoblotted with anti-Csk (C-20). Csk activity was measured in immunoprecipitates by determining 32P incorporated into poly (Glu,Tyr) 4:1 kinase substrate (Sigma-Aldrich) after 5 minutes at 37°C in 50 μL kinase buffer containing 3mM MnCl2, 200 μg/mL poly GluTyr, 2 μCi γ32P-ATP (3000 Ci/mmol), 50μM ATP, 50mM Tris, pH 7.4 as described.20 32P incorporation was determined by acid precipitation of the peptide substrate and scintillation counting of triplicates.
pp60Src was immunoprecipitated with 4 μg anti-Src (GD11; Upstate Biotechnology) from lysates of cells treated as described. Src activity was evaluated using Src assay kit (17-131; Upstate Biotechnology). Replicate immunoprecipitates were electrophoresed and immunoblotted with anti-Src (SRC 2; Santa Cruz Biotechnology).
Protein phosphorylation
Cells treated with or without 20 μg/mL forskolin/250μM dibutyryl cAMP or vehicle control (0.4% DMSO) for 15, 30, or 60 minutes at 37°C were lysed in RIPA buffer. 50 μg cell lysates were immunoblotted with anti-Src (pY529; Biosource International) and anti-Src (Src 2; Santa Cruz Biotechnology). Lysates (500 μg) were incubated with M-450 Dynabeads (DYNAL) coated with 4-5 μg anti-Csk (C-20; Santa Cruz Biotechnology) for 3 hours at 4°C. Immune complexes were immunoblotted with anti-phosphotyrosine (4G10; Upstate Biotechnology), anti-phosphoserine (7F12 and 16B4; Biomol), or anti-Csk (Csk C-20; Santa Cruz Biotechnology).
Cell-cell adhesion
Cells were grown to near confluence on vitronectin-coated plates, then treated with medium, 20 μg/mL forskolin (Biomol), 0.4% DMSO (vehicle control), or 10μM PP2 or PP3 (Calbiochem) for 1 hour. Cells were then incubated in 0.025% trypsin containing 0.27mM EDTA (total cell number) or 1mM CaCl2 (clustered cell number) for 10 minutes. The ratio of cells in multicellular clusters/total cells was determined and calculated as percent cells exhibiting intercellular adhesion (percent adherent cells). Endothelial monolayers were also immunostained with fluorescein isothiocyanate–conjugated anti–VE-cadherin and photographed using a Nikon Eclipse TE2000 microscope with a Cool Snap HQ digital camera (Roper Scientific GmbH), under illumination from a Xenon 75 Watt lamp using a Nikon Plan Apo 40×/0.95 NA or 60×/1.4 NA oil objective. Images were processed using MetaMorph software Version 6.3r5 (Molecular Devices). The ratio of cells in multicellular clusters/total cells was determined and calculated as percent cells exhibiting intercellular adhesion (percent adherent cells).
VE-cadherin phosphorylation
VE-cadherin phosphorylation was measured as follows: HUVECs were serum-starved overnight, stimulated with culture medium containing 200 ng/mL VEGF-A together with 0.4% DMSO (vehicle control), dibutyryl cAMP (500μM) plus forskolin (20 μg/mL), 10μM PP2, or 10μM PP3 for 15 minutes at 37°C, incubated with pervanadate to prevent dephosphorylation (100μM vanadate plus 200μM hydrogen peroxide) for 7 minutes at 37°C, then lysed in RIPA buffer. Lysates (400-600 μg) were incubated with 40 μL of protein G agarose (Santa Cruz Biotechnology) coated with 2 μg anti–VE-cadherin (C-19; Santa Cruz Biotechnology) for 3 hours at 4°C. Immune complexes were washed 3× in lysis buffer and immunoblotted with anti-phosphotyrosine (4G10; Upstate Biotechnology), anti–VE-cadherin, (C-19; Santa Cruz Biotechnology) or anti–β-catenin antibodies (E-5; Santa Cruz Biotechnology).
siRNA transfections
A pool of 4 siRNA duplexes targeting human pp60Src (GenBank accession no. NM_005417) or human Csk as well as a matched nonspecific siRNA pool were purchased from Dharmacon. HUVECs were transfected with siRNAs using the Amaxa Nucleofection System, following the manufacturer's protocols. Working concentration of siRNA was 800nM. Electroporated HUVECs were cultured in EGM (Cambrex) at a density of 30 000 cells/mL for 48 hours before being immunostained with fluorescein isothiocyanate–conjugated anti–VE-cadherin antibody.
Results
PKA activation suppresses cell migration in vitro and angiogenesis in vivo
Angiogenesis is regulated by positive and negative signals that control endothelial cell proliferation, survival, migration, and cell-cell adhesion that culminate in vascular sprouting. While VEGF and other stimuli initiate sprouting, Dll4/Notch and other naturally occurring stimuli play important roles in modulating sprouting.1,2 Naturally occurring inhibitors of sprouting include thrombospondin, endostatin, angiostatin, PTH/PTHrP, prostaglandin E2, retinoic acid, epinephrine, and CXCL10.11-14 Several of these natural angiogenesis inhibitors activate Gαs GPCRs and PKA.11-14 We previously observed that PTH and PTHrP suppress angiogenesis in a PKA-dependent manner.11 We evaluated the general effect of GsPCR ligands and PKA on angiogenesis in chick chorioallantoic membranes (CAMs). Both PTHrP and isoproterenol, a ligand for β-adrenergic receptor, inhibit angiogenesis (Figure 1A-B). This inhibition could be reversed by ectopic expression of a dominant negative PKA isoform (mutant regulatory subunit I; Figure 1C). Furthermore, these effects could be reproduced by cell permeable dibutyryl cAMP, which inhibited VEGF-A and bFGF-mediated angiogenesis (Figure 1D).
PKA activation suppresses angiogenesis. (A) Mean vessel branchpoints ± SEM in CAMs of 10-day-old chicken embryos stimulated with saline or bFGF and topically treated with saline, 20μM PTHrP, calcitonin gene related peptide (CGRP), or isoproterenol, n = 10, *P < .05. (B) Images of CAMs stimulated with saline or bFGF and treated with saline, PTHrP, or isoproterenol. (C) Mean vessel branchpoints ± SEM in chorioallantoic membranes of 10-day–old chicken embryos stimulated with saline or bFGF, transfected with N1-GFP or pcDNA 3.1 V5/his dnPKA (dominant negative PKA = mutationally inactive PKA regulatory subunit I) as previously described10 and topically treated with saline or PTHrP, n = 10, *P < .05. (D) Mean vessel branchpoints ± SEM in chorioallantoic membranes of 10-day-old chicken embryos stimulated with saline, VEGF-A or bFGF, and topically treated with dibutyryl cAMP or saline, n = 10, *P < .05. (E) Images of 32 hpf transgenic GFP-expressing zebrafish larvae in vasculature [TG(fli1:EGFP1)] after treatment for 24 hours with forskolin or H89, a PKA inhibitor. DA (dorsal aorta), PCV (posterior cardinal vein), ISVs (intersegmental vessels), and DLAVs (dorsal longitudinal anastomotic vessels) are indicated for control-treated embryos.
PKA activation suppresses angiogenesis. (A) Mean vessel branchpoints ± SEM in CAMs of 10-day-old chicken embryos stimulated with saline or bFGF and topically treated with saline, 20μM PTHrP, calcitonin gene related peptide (CGRP), or isoproterenol, n = 10, *P < .05. (B) Images of CAMs stimulated with saline or bFGF and treated with saline, PTHrP, or isoproterenol. (C) Mean vessel branchpoints ± SEM in chorioallantoic membranes of 10-day–old chicken embryos stimulated with saline or bFGF, transfected with N1-GFP or pcDNA 3.1 V5/his dnPKA (dominant negative PKA = mutationally inactive PKA regulatory subunit I) as previously described10 and topically treated with saline or PTHrP, n = 10, *P < .05. (D) Mean vessel branchpoints ± SEM in chorioallantoic membranes of 10-day-old chicken embryos stimulated with saline, VEGF-A or bFGF, and topically treated with dibutyryl cAMP or saline, n = 10, *P < .05. (E) Images of 32 hpf transgenic GFP-expressing zebrafish larvae in vasculature [TG(fli1:EGFP1)] after treatment for 24 hours with forskolin or H89, a PKA inhibitor. DA (dorsal aorta), PCV (posterior cardinal vein), ISVs (intersegmental vessels), and DLAVs (dorsal longitudinal anastomotic vessels) are indicated for control-treated embryos.
To determine whether PKA regulates vascular sprouting during embryonic development, we treated optically transparent transgenic zebrafish larvae expressing green fluorescent protein (GFP) in vasculature [Tg(fli1:EGFP)] at 18 hours postfertilization (hpf; when intersegmental blood vessels start to form) for 24 hours with increasing concentrations of the PKA activator forskolin or the selective PKA inhibitor H89. In control embryos, the dorsal aorta (DA), posterior cardinal vein (PCV), intersegmental vessels (ISVs), and the dorsal longitudinal anastomotic vessels (DLAVs) are well formed (Figure 1E and supplemental Videos 1-2). In addition, the DA and PCV are forming lumens at stage, as observed in supplemental Videos 1 and 2. However, in forskolin-treated embryos, the DA and PCV are denser and more cellular than in control embryos and lumen formation is inhibited (Figure 1E and supplemental Videos 3-4). Most significantly, the DLAVs are incompletely formed as some intersegmental vessel fail to migrate completely toward the forming DLAV. (Figure 1E and supplemental Videos 3-4). The failure of DLAV to form properly and failure of the DA and PCV arteries to properly form lumens indicates that forskolin inhibits endothelial cell migration and stimulates intercellular adhesion such that vessels fail to extend or sprout. In contrast, treatment with H89, the selective PKA inhibitor, stimulates excessive sprouting. The DA, PCV, SA, and SV as well DLAVs form normally, but excessive vascular sprouting is observed from the DA and PCV (Figure 1E arrowheads). These results suggest that PKA plays an important role in the regulation of blood vessel morphogenesis by controlling vascular sprouting and suggests that GPCRs that regulate PKA may play important roles in vascular morphogenesis.
To decipher the mechanisms by which PKA regulates vascular remodeling, we tested the effect of PKA activation and inhibition in vitro. We first evaluated the effect of PKA activators on endothelial cell invasion in Matrigel in vitro (Figure 2A). In this assay, endothelial cells form a network of interconnecting branches, called tubes or vessels, in response to endothelial growth factors such as VEGF-A. To quantify the extent of invasion and the number of these structures formed by endothelial cells, we quantified the number of these branchpoints20 in cultures with similar numbers of cells, as determined by counting DAPI (4′,6-diamidino-2-phenylindole)–stained nuclei. Forskolin potently inhibited Matrigel invasion and formation of interconnected networks of endothelial cells (Figure 2A-B). As vessel formation is dependent on a balance between endothelial cell-cell adhesion and cell migration, we quantified the effect of each treatment on the number of cells within the branchpoints. We observed a 12-fold increase in the number of endothelial cells in branchpoints of cultures treated with forskolin (Figure 2A,C), suggesting that activators of PKA stimulate endothelial cell-cell adhesion and/or inhibit endothelial cell migration.
PKA promotes VE-cadherin–mediated endothelial cell-cell adhesion. (A) Endothelial cell tube formation in Matrigel was measured in the presence of culture medium (untreated), culture medium plus vehicle control (DMSO), or culture medium plus forskolin. Brightfield and fluorescence (4′,6-diamidino-2-phenylindole) images of Matrigel cultures are shown. (B) Quantification of vessel-like branchpoints per 100× microscopic field ± SEM formed by the network of tubes in each treatment condition, *P = .0001. (C) Quantification of cell number per branchpoint ± SEM in each treatment condition, *P = .005. (D) VE-cadherin immunostaining (green) of N1-GFP, pcDNA3.1V5/His dnPKA, and pcDNA3.1V5/His PKACat transfected endothelial cells in the absence (−forskolin) or presence of 20 μg/mL forskolin (+forskolin). (E) Percent adherent cells ± SEM in cells from D, *P < .05. Inset: Western blotting of transfected cells for expression of His-tagged PKA transgenes. (F) Top images: endothelial cell tube formation in Matrigel cultures in the presence of vehicle control (DMSO), forskolin, and PTHrP. Bottom images: VE-cadherin cell-cell contact formation in the presence of DMSO, forskolin, or PTHrP. (G) Cell number/branchpoint in DMSO, forskolin, or PTHrP-treated Matrigel cultures. (H) Percent adherent cells ± SEM in cells treated with DMSO, forskolin, PTHrP, or isoproterenol, *P < .05.
PKA promotes VE-cadherin–mediated endothelial cell-cell adhesion. (A) Endothelial cell tube formation in Matrigel was measured in the presence of culture medium (untreated), culture medium plus vehicle control (DMSO), or culture medium plus forskolin. Brightfield and fluorescence (4′,6-diamidino-2-phenylindole) images of Matrigel cultures are shown. (B) Quantification of vessel-like branchpoints per 100× microscopic field ± SEM formed by the network of tubes in each treatment condition, *P = .0001. (C) Quantification of cell number per branchpoint ± SEM in each treatment condition, *P = .005. (D) VE-cadherin immunostaining (green) of N1-GFP, pcDNA3.1V5/His dnPKA, and pcDNA3.1V5/His PKACat transfected endothelial cells in the absence (−forskolin) or presence of 20 μg/mL forskolin (+forskolin). (E) Percent adherent cells ± SEM in cells from D, *P < .05. Inset: Western blotting of transfected cells for expression of His-tagged PKA transgenes. (F) Top images: endothelial cell tube formation in Matrigel cultures in the presence of vehicle control (DMSO), forskolin, and PTHrP. Bottom images: VE-cadherin cell-cell contact formation in the presence of DMSO, forskolin, or PTHrP. (G) Cell number/branchpoint in DMSO, forskolin, or PTHrP-treated Matrigel cultures. (H) Percent adherent cells ± SEM in cells treated with DMSO, forskolin, PTHrP, or isoproterenol, *P < .05.
PKA activation stimulates endothelial cell-cell adhesion
To determine whether PKA regulates cell-cell adhesion or cell migration during vessel formation, we evaluated the effect of forskolin on VEGF-stimulated intercellular adhesion in vitro and in vivo. Endothelial cells were transfected with a control GFP construct, with a dominant negative PKA (an inactivated R1 regulatory subunit)–expressing construct15 or with a catalytic (active) subunit PKA-expressing construct and then were treated with or without forskolin. Forskolin rapidly promoted cell-cell adhesion in control-transfected cells, as measured by the formation of VE-cadherin adherens junctions between adjacent endothelial cells in culture (Figure 2D-E and supplemental Figure 1A,C) or by measurement of intercellular distances between endothelial cells (supplemental Figure 1D). Treatment of endothelial cell monolayers with forskolin rapidly stimulated VE-cadherin localization at cell boundaries (Figure 2D and supplemental Figure 1C), decreased intercellular distances (supplemental Figure 1D), and increased intercellular adhesion between endothelial cells in vitro (Figure 2E and supplemental Figure 1A). However, expression of dominant negative PKA (dnPKA) prevented forskolin-mediated intercellular adhesion and VE-cadherin zipper formation (Figure 2D-F), while expression of the catalytic subunit of PKA (PKAcat) stimulated VE-cadherin zipper formation and cell-cell adhesion in the absence of forskolin (Figure 2D-F). These results indicate that PKA promotes endothelial cell-cell adhesion.
Ligands for the Gs GPCRs PTHR1 and β2-adrenergic receptor, which activate PKA, also stimulated endothelial cell-cell adhesion. Like forskolin, PTHrP and isoproterenol inhibited endothelial tube formation by increasing in the number of endothelial cells within branchpoints of treated cultures and promoted increased VE-cadherin zipper formation in endothelial cell monolayers (Figure 2F-G). These results indicate that physiologic regulators of PKA suppress endothelial cell invasion while stimulating endothelial cell-cell adhesion.
PKA activation stimulates endothelial cell-cell adhesion in vivo
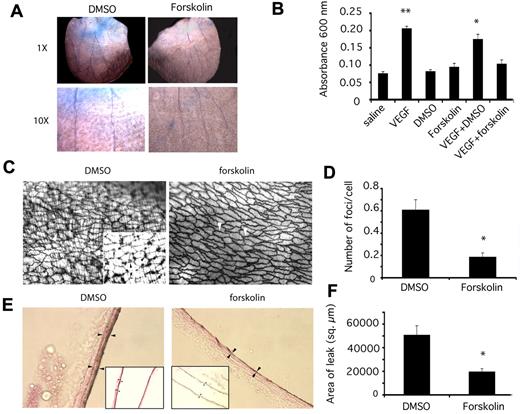
To investigate whether PKA might also regulate endothelial cell-cell adhesion in vivo, we evaluated the effect of forskolin on VEGF-A–mediated vascular leak in vivo. VEGF-A was first described as vascular permeability factor, as it decreased intercellular adhesion between endothelial cells and induced leakage of plasma and cells into the subendothelial cell space.21 To determine whether forskolin affects endothelial cell-cell adhesion in vivo, we evaluated its effects in a model of VEGF-induced vascular leakage using a Miles assay.21,22 In this model, vascular leak was stimulated by intradermal injection of VEGF-A in the ear skin. Control animals were injected in the alternate ear with saline. Animals were then injected intravascularly with Evans Blue dye, a di-azo dye that binds to albumin, followed by forskolin or vehicle control. When intercellular junctions between endothelial cells are disrupted, as by VEGF-A signaling, Evans Blue-albumin complexes deposit in the subendothelial space and tissue appears blue. VEGF-A stimulated significant leakage of Evans Blue from the vasculature, while saline did not (Figure 3A-B; *P = .00001). Forskolin, but not vehicle control (DMSO), inhibited VEGF-A–mediated vascular leak (Figure 3A-B; **P = .012), while forskolin and DMSO in the absence of VEGF-A had no effect on vascular leak. These studies indicate that activators of PKA suppresses the effects of VEGF-A on vascular leak. As vascular leak has previously been shown to result from decreased endothelial cell-cell adhesion, our results suggest that PKA stimulates endothelial cell-cell adhesion in vivo.
PKA promotes endothelial cell-cell adhesion in vivo and suppresses vascular leak. (A) Images of mouse ears after Evans Blue dye leakage at 1× and 10× following stimulation by VEGF and treatment with vehicle control (2% DMSO) or forskolin. (B) Quantification of vascular leak by dye extraction and measurement of Evans Blue absorbance at 600 nm ± SEM after intradermal injection of VEGF or saline and treatment with 2% DMSO or 100μM forskolin, n = 10, **P = .00 001, *P = .013, in comparison to saline. (C) Brightfield images at 200× and 1000× (inset) of silver nitrate–stained aortas that were stimulated with VEGF and treated with DMSO or forskolin. Silver nitrate foci at endothelial cell junctions are indicated by white arrowheads or by black arrowheads in the inset. Bands of vascular smooth muscle surrounding the endothelium are also outlined. (D) Average number ± SEM of silver nitrate foci/cell in 200× microscopic fields in DMSO and forskolin treated vessels, n = 6, *P = .00 001. (E) Brightfield images at 600× and 200× (inset) of silver nitrate stained carotid arteries that were stimulated with VEGF and treated with DMSO or forskolin. Arrowheads indicate areas of silver nitrate stain. (F) Average area of silver nitrate staining ± SEM in 600× microscopic fields in DMSO and forskolin treated vessels, n = 6, *P = .004.
PKA promotes endothelial cell-cell adhesion in vivo and suppresses vascular leak. (A) Images of mouse ears after Evans Blue dye leakage at 1× and 10× following stimulation by VEGF and treatment with vehicle control (2% DMSO) or forskolin. (B) Quantification of vascular leak by dye extraction and measurement of Evans Blue absorbance at 600 nm ± SEM after intradermal injection of VEGF or saline and treatment with 2% DMSO or 100μM forskolin, n = 10, **P = .00 001, *P = .013, in comparison to saline. (C) Brightfield images at 200× and 1000× (inset) of silver nitrate–stained aortas that were stimulated with VEGF and treated with DMSO or forskolin. Silver nitrate foci at endothelial cell junctions are indicated by white arrowheads or by black arrowheads in the inset. Bands of vascular smooth muscle surrounding the endothelium are also outlined. (D) Average number ± SEM of silver nitrate foci/cell in 200× microscopic fields in DMSO and forskolin treated vessels, n = 6, *P = .00 001. (E) Brightfield images at 600× and 200× (inset) of silver nitrate stained carotid arteries that were stimulated with VEGF and treated with DMSO or forskolin. Arrowheads indicate areas of silver nitrate stain. (F) Average area of silver nitrate staining ± SEM in 600× microscopic fields in DMSO and forskolin treated vessels, n = 6, *P = .004.
To evaluate further the effects of PKA activity on endothelial cell-cell adhesion in vivo, we stimulated animals by intravascular injection of VEGF and treated with forskolin or vehicle control (Figure 3C-F). Immediately after sacrifice, animals were perfused with silver nitrate, which deposits in the inter- and subendothelial cell spaces in blood vessels. High-powered light microscopy of excised aortas revealed that VEGF-A induced leakage of silver nitrate into the subendothelial cell space (Figure 3C; DMSO). Large focal areas of silver nitrate were deposited at endothelial cell junctions (Figure 3C inset); bands of vascular smooth muscle surrounding the endothelium were also outlined when aortas were stimulated with VEGF-A and treated with vehicle control (VEGF plus DMSO; Figure 3C). In contrast, few such foci were observed in aortas from untreated animals or in animals treated with VEGF and forskolin (VEGF plus forskolin; Figure 3C). Instead, silver nitrate lightly outlined the borders between endothelial cells, but did not leak between cells (arrowheads, VEGF plus forskolin; Figure 3C). The average number of foci outlined by silver nitrate in forskolin-treated animals was 3-fold less than in control-treated animals (Figure 3D; *P = .00001). In addition, the area of silver staining in carotid arteries was significantly less in forskolin-treated animals than in control-treated animals (Figure 3E-F). The amount of silver deposited in carotid arteries in forskolin-treated arteries was at least 2.5× less than in control treated arteries (Figure 3E-F; *P = .004). Together with in vitro studies, these studies indicate that PKA stimulates endothelial cell-cell adhesion in vitro and in vivo even in the presence of VEGF-A.
PKA activation inhibits endothelial cell migration
Our previous studies showed that endothelial cell migration and angiogenesis could be inhibited by hormones such as PTH and PTHrP that activate PKA by stimulating Gs coupled receptors.11 To determine in greater detail the mechanisms by which PKA regulates cell migration, we examined the effects of PKA activation on cell migration.
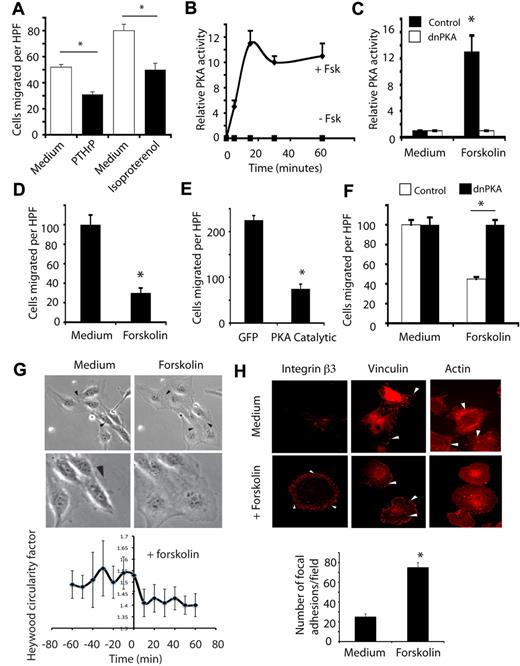
Exposure of primary endothelial cells to either PTHrP or isoproterenol inhibited endothelial cell migation on vitronectin substrates (Figure 4A). We further evaluated the role of PKA in cell migration with forskolin. Forskolin activated PKA in cultured endothelial cells, reaching maximal activation within 10-15 minutes, as determined by enzymatic assays of endothelial cell extracts (Figure 4B). This activation could be completely inhibited by expression of the mutationally inactivated PKA regulatory subunit I (dnPKA; Figure 4C). Forskolin rapidly and potently inhibited cell migration as determined by performing Boyden chamber type migration assays (Figure 4D) or scratch wound healing-type migration assays (not shown). Importantly, these effects could be reproduced by ectopic expression of the PKA catalytic subunit (Figure 4E). The inhibitory effect of forskolin on cell migration could be reversed by expression of dominant negative, mutant PKA regulatory subunit (dnPKA) indicating that PKA directly regulates endothelial cell migration (Figure 4F).
PKA inhibits endothelial cell migration. (A) Number of endothelial cells ± SEM migrating on vitronectin in serum-free culture medium or in culture medium containing PTHrP or isoproterenol, *P < .05. (B) Relative levels of PKA activity ± SEM in endothelial cells treated with culture medium or 20 μg/mL forskolin, *P < .05. (C) Relative levels of activity ± SEM in ECs after transfection with pcDNA 3.1 V5/his-dominant negative PKA (dnPKA) or N1GFP (GFP) with or without treatment with forskolin, *P < .05. (D) Number of endothelial cells ± SEM migrating on vitronectin in the absence (filled bars) or presence of forskolin, *P < .05. (E) Number of endothelial cells ± SEM migrating on vitronectin after transfection with pcDNA 3.1 V5/his-PKA catalytic subunit and N1GFP (PKA Catalytic) or N1GFP (GFP) vectors, *P < .05. (F) Number of endothelial cells ± SEM migrating on vitronectin after transfection with pcDNA 3.1 V5/his-RImut (dnPKA) or N1GFP treated with culture medium or forskolin, *P < .05. (G) Freeze frame photographs at 200× and 400× from time-lapse microscopy of endothelial cells after addition of medium or forskolin. Arrowheads indicate cell edges. See also the supplemental Videos. Graph: Heywood circularity factor calculated at 10-minute intervals for 60 minutes before and after forskolin treatment. (H) Endothelial cells treated with or without forskolin were attached to vitronectin-coated coverslips for 60 minutes and stained with rhodamine-phalloidin (actin), anti-vinculin (vinculin), or anti-integrin αvβ3 integrin β3. Arrowheads indicate focal adhesions. Graph: number of vinculin-containing focal adhesions in cells treated with or without forskolin.
PKA inhibits endothelial cell migration. (A) Number of endothelial cells ± SEM migrating on vitronectin in serum-free culture medium or in culture medium containing PTHrP or isoproterenol, *P < .05. (B) Relative levels of PKA activity ± SEM in endothelial cells treated with culture medium or 20 μg/mL forskolin, *P < .05. (C) Relative levels of activity ± SEM in ECs after transfection with pcDNA 3.1 V5/his-dominant negative PKA (dnPKA) or N1GFP (GFP) with or without treatment with forskolin, *P < .05. (D) Number of endothelial cells ± SEM migrating on vitronectin in the absence (filled bars) or presence of forskolin, *P < .05. (E) Number of endothelial cells ± SEM migrating on vitronectin after transfection with pcDNA 3.1 V5/his-PKA catalytic subunit and N1GFP (PKA Catalytic) or N1GFP (GFP) vectors, *P < .05. (F) Number of endothelial cells ± SEM migrating on vitronectin after transfection with pcDNA 3.1 V5/his-RImut (dnPKA) or N1GFP treated with culture medium or forskolin, *P < .05. (G) Freeze frame photographs at 200× and 400× from time-lapse microscopy of endothelial cells after addition of medium or forskolin. Arrowheads indicate cell edges. See also the supplemental Videos. Graph: Heywood circularity factor calculated at 10-minute intervals for 60 minutes before and after forskolin treatment. (H) Endothelial cells treated with or without forskolin were attached to vitronectin-coated coverslips for 60 minutes and stained with rhodamine-phalloidin (actin), anti-vinculin (vinculin), or anti-integrin αvβ3 integrin β3. Arrowheads indicate focal adhesions. Graph: number of vinculin-containing focal adhesions in cells treated with or without forskolin.
PKA stimulates loss of cell polarity and increased focal adhesion formation
To determine the mechanisms by which PKA inhibits cell migration and stimulates intercellular adhesion, we performed time-lapse microscopy of migrating endothelial cells in the presence and absence of activators of PKA. These studies show that activation of PKA rapidly inhibits endothelial cell migration by inhibiting forward momentum, increasing cell spreading and decreasing cell polarity (Figure 4G and supplemental Videos 5-6). Within 10 minutes of exposure to forskolin, cell migration was completely blocked and cell shape was dramatically altered. Cells stably flattened against the substrate and cell edges touched, suggesting increased cell-substrate and cell-cell adhesion. To quantify collective cell movement, the distance individual cell nuclei traveled over the 1-hour period was measured. While untreated cells moved significant distances, cells with activators of PKA did not significantly move after treatment (supplemental Figure 2A and supplemental Videos 5-6). To quantify cell polarity, the degree of cell polarity was determined by comparing the perimeter of individual cells to that of a circle with the same area (the Heywood Circularity Factor). Our studies show that forskolin treatment rapidly and sustainably inhibited cell polarization and promoted cell flattening (Figure 4G).
These studies on the effect of PKA activation on cell shape suggested that endothelial cells might cease movement because of a decreased ability to detach from the substrate. Therefore, we examined the effects of PKA activation on the actin cytoskeleton. While untreated endothelial cells were spindle-shaped and exhibited actin fibrils that traversed the cell (stress fibers), forskolin-treated cells were nonpolar and exhibited only cortical actin filaments (Figure 4H). While untreated endothelial cells exhibited a few large vinculin and β3-integrin–containing focal adhesions at the leading and trailing edges of the cell, PKA activation stimulated a large increase in the number and a decrease in the size of vinculin or β3-integrin–containing focal adhesions plaques (Figure 4H). The distribution of these structures was also altered; upon PKA activation, focal adhesions were distributed around the entire cell periphery rather than at the leading and trailing edges as in untreated cells (Figure 4H). These studies indicate that PKA activation inhibits cell migration by substantially stimulating focal adhesion formation or preventing focal adhesion remodeling at the cell periphery over the entire cell perimeter. Our results also suggest that this lack of polarity in focal adhesion structures prevents directional cell movement. These studies indicate that PKA activation inhibits cell migration by regulating the molecules that control remodeling of the cytoskeleton.
PKA regulates pp60Src
Our studies show that PKA activation in endothelial cells suppresses cell migration and stimulates cell-cell adhesion. Importantly, another key cellular kinase that has also been shown to oppositely regulate cell adhesion and cell migration is the tyrosine kinase pp60Src. Src plays a role in promoting the epithelial to mesenchymal transition by inhibiting E-cadherin function and by stimulating cell migration.23,24 In addition, Src has also been shown to inhibit endothelial cell adherens junction formation and to thereby promote vascular leak by phosphorylating VE-cadherin and β-catenin.23,25 Src also promotes focal adhesion turnover and cell migration.9,26 As PKA and Src appear to have opposing actions on the same processes, we asked whether PKA negatively regulates Src function.
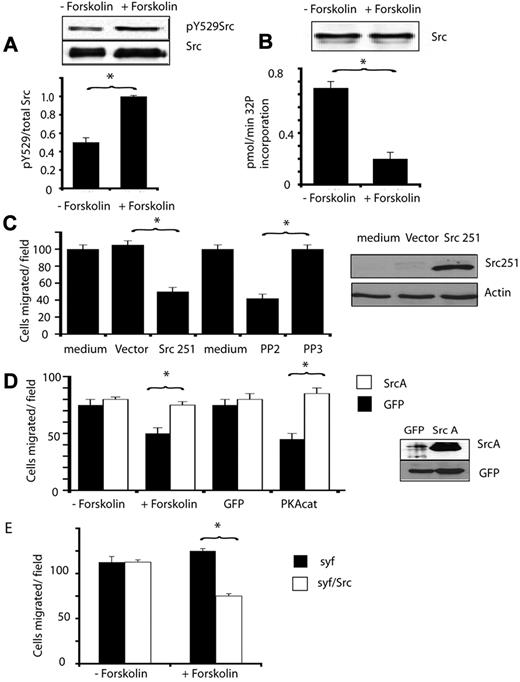
We therefore determined whether PKA modulates Src activity in endothelial cells by first determining whether PKA affects Src phosphorylation. Src is negatively regulated by tyrosine phosphorylation on the C-terminal tyrosine 529. Treatment of endothelial cells with forskolin rapidly (within 15 minutes) stimulated phosphorylation of Src on tyrosine 529 (Figure 5A) and inhibited the ability of immunoprecipated Src to phosphorylate the Src substrate poly (Glu,Tyr) 4:1 (Figure 5B). Src activity remained suppressed for at least 1 hour after stimulation by forskolin. These results indicate that PKA suppresses Src activation.
PKA inhibits endothelial cell migration by suppressing Src activity. (A) Anti-Src pY529 and anti-total Src immunoblots of lysates from endothelial cells treated with or without forskolin (+forskolin). pY529Src/total Src ratio was determined by densitometry, *P < .05. (B) Graph: 32P incorporation into a Src substrate (KVEKIGEGTYGVVYK) by Src immunoprecipitated from cells treated with and without forskolin, expressed as pmol32P incorporated per minute ± SEM, *P < .05. Blot: Loading control showing Src immunoprecipitates blotted with anti-Src antibodies. (C) Mean number of endothelial cells migrating ± SEM after transduction with control virus (CA10) or virus expressing truncated Src, Src251 (CA10Src251), or after treatment with culture medium or medium containing 10μM PP2 (Src inhibitor) or 10μM PP3 (negative control), *P < .05. Blot: Src251 expression demonstrated by immunoblotting of cell lysates with anti-Src antibodies. (D) Mean number ± SEM of migrated control transfected endothelial cells (dark bars) or SrcY529A transfected endothelial cells (SrcA, white bars) treated with or without forskolin or transfected with PKACat or GFP. Blot: Transfected cell lysates immunoblotted with anti-Src and anti-GFP antibodies. (E) Mean number of migrating src-fyn-yes−/− fibroblasts (syf) and syf fibroblasts stably expressing Src (syf/Src) that were treated with medium (−forskolin) or 20 μg/mL forskolin (+forskolin). *P < .05.
PKA inhibits endothelial cell migration by suppressing Src activity. (A) Anti-Src pY529 and anti-total Src immunoblots of lysates from endothelial cells treated with or without forskolin (+forskolin). pY529Src/total Src ratio was determined by densitometry, *P < .05. (B) Graph: 32P incorporation into a Src substrate (KVEKIGEGTYGVVYK) by Src immunoprecipitated from cells treated with and without forskolin, expressed as pmol32P incorporated per minute ± SEM, *P < .05. Blot: Loading control showing Src immunoprecipitates blotted with anti-Src antibodies. (C) Mean number of endothelial cells migrating ± SEM after transduction with control virus (CA10) or virus expressing truncated Src, Src251 (CA10Src251), or after treatment with culture medium or medium containing 10μM PP2 (Src inhibitor) or 10μM PP3 (negative control), *P < .05. Blot: Src251 expression demonstrated by immunoblotting of cell lysates with anti-Src antibodies. (D) Mean number ± SEM of migrated control transfected endothelial cells (dark bars) or SrcY529A transfected endothelial cells (SrcA, white bars) treated with or without forskolin or transfected with PKACat or GFP. Blot: Transfected cell lysates immunoblotted with anti-Src and anti-GFP antibodies. (E) Mean number of migrating src-fyn-yes−/− fibroblasts (syf) and syf fibroblasts stably expressing Src (syf/Src) that were treated with medium (−forskolin) or 20 μg/mL forskolin (+forskolin). *P < .05.
To determine whether Src activity is required for endothelial cell migration, we expressed dominant negative Src (SrcΔ251, a truncated form of Src lacking the kinase domain) or the control transgene GFP in endothelial cells. SrcΔ251 but not GFP expression inhibited endothelial cell migration (Figure 5C). Similarly, treatment of endothelial cells with the pharmacologic Src inhibitor PP2 also blocked cell migration while treatment with the control substance PP3 had no effect (Figure 5C). To determine whether the PKA-mediated inhibition of cell migration depends on Src inhibition, we transfected endothelial cells with constitutively active Src, SrcA (SrcY529A), or with the control transgene GFP. Expression of activated Src blocked the inhibitory effects of forskolin on cell migration (Figure 5D). Expression of activated Src also blocked the effects of expression of the PKA catalytic subunit gene (Figure 5D), confirming that PKA suppression of Src activity inhibits cell migration. Finally, to test the dependence of PKA-mediated inhibition of cell migration on Src, we evaluated the effect of forskolin on cell migration in cells genetically lacking Src. Notably, fibroblasts derived from src/fyn/yes−/− mice (syf−/−),27 deficient mice are refractory to forskolin-mediated inhibition of cell migration (Figure 5E). In contrast, reexpression of Src in syf−/− cells (syf/Src) rendered them susceptible to inhibition by PKA activators (Figure 5E). Taken together, these studies indicate that PKA activation inhibits Src and that Src is an intermediate in the pathway by which PKA regulates endothelial cell migration.
PKA inhibits Src phosphorylation of VE-cadherin
During angiogenesis, endothelial cells loosen intercellular adhesive contacts and migrate. Src kinase may promote both of these events, by oppositely regulating intercellular adhesion and cell migration. Src phosphorylates cadherins, thereby preventing their association with catenins, interfering with cadherin clustering and cell-cell adhesion in vitro and in vivo.23,24 In fact, inhibition of Src activity blocks these phosphorylation events and promotes endothelial cell intercellular adhesion. Thus, Src activity plays a key role in regulating the migratory phenotype by inhibiting cell-cell adhesion and promoting cell migration.
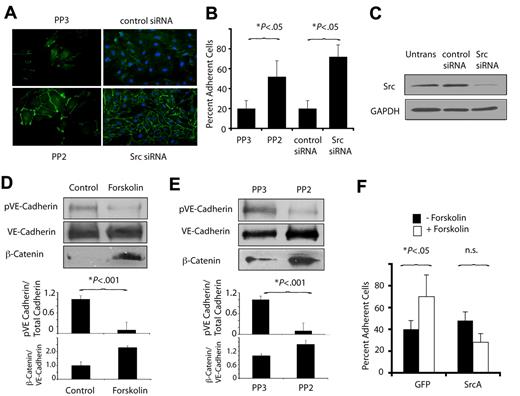
Our studies have shown that PKA activity inhibits endothelial cell migration and also promotes cell-cell adhesion. As PKA inhibition of Src activity suppresses cell migration, we asked whether PKA might promote cell-cell adhesion by inhibiting Src function. We found that endothelial cell-cell adhesion and VE-cadherin zipper formation were enhanced by the Src inhibitor PP2 or by knockdown of Src using siRNA (Figure 6A-C). As Src directly phosphorylates and inhibits VE-cadherin,24 we asked whether activation of PKA could inhibit tyrosine phosphorylation of VE-cadherin. Activation of PKA in endothelial cells by exposure to forskolin, as well as inhibition of Src by PP2, inhibited VE-cadherin phosphorylation and blocked β-catenin dissociation from adherens junctions (Figure 6D-E). These studies indicate that PKA-mediated inhibition of Src blocks tyrosine phosphorylation of VE-cadherin.
PKA and Src oppositely regulate VE-cadherin function. (A) Anti–VE-cadherin immunostaining of endothelial cell monolayers cultured in the presence of the Src inhibitor PP2 (PP2) or chemically inert control PP3 (PP3) and of endothelial cells transfected with Src siRNA or control siRNA. (B) Quantification of the mean ± SEM percent adherent cells in A. (C) Immunoblotting to detect Src of endothelial cells expressing Src siRNA or scrambled siRNA. (D) Immunoblot of phosphoVE-cadherin, VE-cadherin, and β-catenin after treatment of VEGF-stimulated endothelial cells with culture medium or forskolin. Graphs: ratios of phosphoVE-cadherin or β-catenin to total VE-cadherin. (E) Immunoblot of phosphoVE-cadherin, VE-cadherin, and β-catenin after treatment of VEGF-stimulated endothelial cells with PP2 or PP3. Graphs: ratios of phosphoVE-cadherin or β-catenin to total VE-cadherin. (F) Cell-cell adhesion of GFP or SrcA transfected EC treated with or without forskolin, expressed as mean ± SEM percent adherent cells.
PKA and Src oppositely regulate VE-cadherin function. (A) Anti–VE-cadherin immunostaining of endothelial cell monolayers cultured in the presence of the Src inhibitor PP2 (PP2) or chemically inert control PP3 (PP3) and of endothelial cells transfected with Src siRNA or control siRNA. (B) Quantification of the mean ± SEM percent adherent cells in A. (C) Immunoblotting to detect Src of endothelial cells expressing Src siRNA or scrambled siRNA. (D) Immunoblot of phosphoVE-cadherin, VE-cadherin, and β-catenin after treatment of VEGF-stimulated endothelial cells with culture medium or forskolin. Graphs: ratios of phosphoVE-cadherin or β-catenin to total VE-cadherin. (E) Immunoblot of phosphoVE-cadherin, VE-cadherin, and β-catenin after treatment of VEGF-stimulated endothelial cells with PP2 or PP3. Graphs: ratios of phosphoVE-cadherin or β-catenin to total VE-cadherin. (F) Cell-cell adhesion of GFP or SrcA transfected EC treated with or without forskolin, expressed as mean ± SEM percent adherent cells.
To determine whether PKA stimulation of VE-cadherin–mediated cell-cell adhesion requires Src inhibition, endothelial cells were transduced with an activated construct of Src, Src A. Src A expression inhibited endothelial cell-cell adhesion and prevented forskolin-induced endothelial cell-cell adhesion (Figure 6F). These studies indicate that PKA-mediated inhibition of Src promotes VE-cadherin–mediated cell-cell adhesion.
PKA-mediated inhibition of migration is Csk-dependent
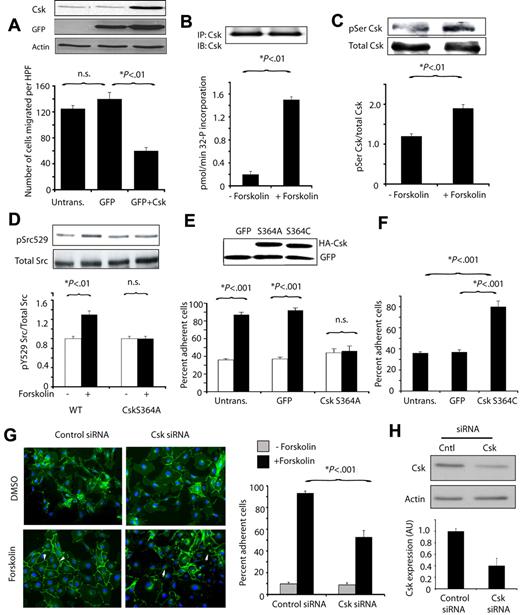
As we found no evidence that PKA directly phosphorylates or modulates the activity of Src (data not shown), we considered whether PKA inhibits cell migration or promotes cell-cell adhesion by activating Csk, which negatively regulates Src by tyrosine 529 phosphorylation. Csk itself can be phosphorylated and activated by PKA in T cells and fibroblasts.28,29 We found that expression of Csk substantially inhibited endothelial cell migration (Figure 7A). PKA promoted Csk activation, as Csk immunoprecipitated from forskolin-treated but not control-treated endothelial cells incorporated radioactive phosphate into a Csk peptide substrate (Figure 7B). Prior studies have indicated that PKA activates Csk by phosphorylation on serine 364.28 We found that PKA activation by forskolin rapidly induced Csk serine phosphorylation in endothelial cells (Figure 7C). These studies thus indicate that PKA can phosphorylate and activate Csk in endothelial cells.
PKA phosphorylates and activates Csk, which suppresses cell migration and promotes cell-cell adhesion. (A) Mean number of endothelial cells migrating on vitronectin ± SEM after transfection with WT Csk or GFP control vector. Blot: Csk, actin, and GFP expression detected by immunoblotting with anti-Csk (C-20), anti-GFP, and anti-actin antibodies. (B) Mean 32P incorporation into the Csk substrate polyGluTyr by immunoprecipitated Csk, expressed as pmol 32P incorporated per minute ± SEM without and with forskolin treatment. Blot: samples of Csk immunoprecipitates immunoblotted with anti-Csk antibodies. (C) Csk immunoprecipitated from cells treated with or without forskolin was immunoblotted with anti-phosphoserine and anti-Csk antibodies. Graph: ratio of serine phosphorylated Csk to total Csk. (D) Immunoblots of phosphoSrcY529 and total Src in lysates from cells expressing GFP or inactive Csk (CskS364A) treated with or without forskolin. Graph: ratio of phosphoSrcY529 to total Src. (E) Mean ± SEM percent adherent cells for endothelial cells transfected with GFP or CskS364A and treated with (black bars) or without (white bars) forskolin. Blot: Lysates of transfected cells from E and F immunoblotted to detect HA-tagged transgenes. (F) Mean ± SEM percent adherent cells for endothelial cells transfected with GFP or activated Csk (CskS364C). (G) Images and graph of mean ± SEM percent adherent cells for endothelial cells transfected with control or Csk siRNA and treated with or without forskolin. (H) Csk expression in cells treated with control or Csk siRNA demonstrated by immunoblotting. Graph: quantification of Csk expression.
PKA phosphorylates and activates Csk, which suppresses cell migration and promotes cell-cell adhesion. (A) Mean number of endothelial cells migrating on vitronectin ± SEM after transfection with WT Csk or GFP control vector. Blot: Csk, actin, and GFP expression detected by immunoblotting with anti-Csk (C-20), anti-GFP, and anti-actin antibodies. (B) Mean 32P incorporation into the Csk substrate polyGluTyr by immunoprecipitated Csk, expressed as pmol 32P incorporated per minute ± SEM without and with forskolin treatment. Blot: samples of Csk immunoprecipitates immunoblotted with anti-Csk antibodies. (C) Csk immunoprecipitated from cells treated with or without forskolin was immunoblotted with anti-phosphoserine and anti-Csk antibodies. Graph: ratio of serine phosphorylated Csk to total Csk. (D) Immunoblots of phosphoSrcY529 and total Src in lysates from cells expressing GFP or inactive Csk (CskS364A) treated with or without forskolin. Graph: ratio of phosphoSrcY529 to total Src. (E) Mean ± SEM percent adherent cells for endothelial cells transfected with GFP or CskS364A and treated with (black bars) or without (white bars) forskolin. Blot: Lysates of transfected cells from E and F immunoblotted to detect HA-tagged transgenes. (F) Mean ± SEM percent adherent cells for endothelial cells transfected with GFP or activated Csk (CskS364C). (G) Images and graph of mean ± SEM percent adherent cells for endothelial cells transfected with control or Csk siRNA and treated with or without forskolin. (H) Csk expression in cells treated with control or Csk siRNA demonstrated by immunoblotting. Graph: quantification of Csk expression.
To determine whether PKA regulates Src in a Csk-dependent manner, we tested the effect of forskolin on Src phosphorylation in endothelial cells. Forskolin stimulated tyrosine phosphorylation of SrcY529 in control transfected endothelial cells but not in endothelial cells transfected with Csk S364A, an inactive Csk variant that cannot be phosphorylated or activated by PKA (Figure 7D).
We next asked whether forskolin induced cell-cell adhesion requires Csk activity. Expression of inactive Csk (CskS364A) inhibited cell-cell adhesion induced by forskolin (Figure 7E), while expression of Csk S364C, a constitutively active Csk variant, promoted cell-cell adhesion (Figure 7F). In addition, expression of Csk S364A also reversed the effects of forskolin on migration (not shown). We also found that siRNA-mediated ablation of Csk expression suppressed forskolin-stimulated endothelial cell-cell adhesion (Figure 7G-H). These studies thus show that activated PKA directly stimulates Csk phosphorylation and activation, and Csk suppresses Src activation. Our studies show that like PKA, Csk oppositely regulates cell migration and endothelial cell-cell adhesion. As Src is constitutively activated and normal vascular sprouting is disrupted in Csk−/− embryos,30-32 these studies indicate that Src and Csk play an important roles in control of angiogenesis.
Activation of PKA suppresses cell migration through its direct regulation of Csk and indirect regulation of Src. In conclusion, these studies reveal that a PKA-driven signaling pathway regulates the switch between migratory and stably adhesive endothelial cell phenotypes.
Discussion
Our studies demonstrate that hormones, growth factors, and other effectors that activate Gαs GPCRs PKA can suppress the transition of endothelial cells to the migratory phenotype by rapidly inhibiting cell migration and stimulating cell-cell adhesion. We demonstrate that PKA activates Csk, which inhibits Src, and that PKA is a critical regulator of invasive cell behavior during angiogenesis.
Recent studies discovered that 2 endothelial cell behaviors regulate angiogenic sprouting from preexisting vessels. Tip endothelial cells exhibit properties of highly invasive, motile cells while stalk endothelial cells exhibit strong cell-cell adhesion and less motile behavior. Dll4 in tip cells interacts with Notch in stalk cells to suppress tip cell induction among stalk cells.1,2 Our observations suggest that Notch/DLL and Gαs GPCR/PKA regulate similar pathways as both suppress migratory behavior while stimulating cell-to-cell adhesion. Blockade of Notch and PKA both lead to excessive embryonic sprouting and excessive stimulation leads to inhibition of sprouting. Our observations suggest that Notch and PKA may regulate angiogenic sprouting through parallel or intersecting pathways.
PKA activity has previously been shown to disrupt normal cell morphology and inhibit stress fiber formation.33 Chemokines, hormones, and other agents, such as PTH, PTHrP, prostaglandin E2, retinoic acid, epinephrine, and CXCL10, suppress endothelial cell migration and inhibit angiogenesis in vivo, in part by activating PKA.11-14 In addition, pharmacologic or genetic activation of PKA directly inhibits cell migration in endothelial cells and other cell types.11-14,16,17 In our studies, PKA activation in endothelial cells stimulates focal adhesion formation and flattens cells, inducing them to assume a circular, nonpolar, nonmigratory shape. Interestingly, PKA RΙα−/− fibroblasts, which exhibit high basal PKA activity, fail to form stress fibers and flatten out to acquire a pancake-like shape.34 Taken together, these results demonstrate that PKA activity inhibits cell migration by modulating the actin cytoskeleton.
The tyrosine kinase Src both promotes cell migration and inhibits cell-cell adhesion.9,23-26,33,34 Src phosphorylates and activates a number of kinases and adaptor proteins such as FAK, Cas, and paxillin that promote cell migration by mediating focal adhesion turnover.9.24 It also phosphorylates and inhibits E-cadherin and VE-cadherin.23,25,35-37 Src-dependent tyrosine phosphorylation of E-cadherin inhibits the association of E-cadherin and its adherens junction partner, β-catenin.24,35-37 This dissociation of β-catenin and E-cadherin prevents E-cadherin-actin cytoskeleton associations and induces movement of β-catenin into the nucleus, where it signals a transition from an epithelial to a mesenchymal phenotype. Thus, Src plays a key role in simultaneously promoting cell migration and inhibiting cell-cell adhesion. By antagonizing Src function, PKA suppresses both endothelial cell migration and promotes endothelial cell intercellular adhesion.
Csk also plays important roles in embryonic development. Csk−/− embryos are early embryonic lethal (E9.5-10.5), exhibit excessive Src activation, and numerous neurologic and vascular defects that include defective vascular sprouting and absent vascular beds.30-32
The studies presented here indicate that PKA promotes cadherin mediated cell-cell adhesion. Our studies show that PKA, through its effects on Csk, inhibits Src, thereby promoting VE-cadherin–mediated cell-cell adhesion and inhibiting vascular permeability in vivo. Previous studies have indicated that PKA promotes endothelial barrier function in vitro, which can be regulated either by adherens junctions or by tight junctions.38,39 Our studies add further insight to the role of PKA in the regulation of endothelial biology. Although other studies have shown that exchange protein activated by cAMP (Epac) plays a role in endothelial cellular responses to cAMP by regulating cell migration and cell-cell adhesion,40 our studies clearly demonstrate the PKA uniquely regulates Src activity, thereby stimulating cell-cell adhesion and inhibiting cell migration.
We have shown that PKA, through its actions on Csk and Src, regulates a switch between cell migration and intercellular adhesion. Our studies suggest that PKA may play a role in suppressing the epithelial to mesenchymal transition. This shift between a stably adherent cell phenotype to an invasive migratory phenotype occurs when cells acquire a new ability to down-regulate intercellular adhesive contacts and to activate cell migration machinery. Epithelial-to-mesenchymal transition–like events occurs in cancer cells, fibroblasts, and endothelial cells.41 The studies presented in this manuscript suggest that PKA may play a general role in regulating the epithelial to mesenchymal transition. Our studies show that PKA activation by specific hormones or chemokines inhibits cell migration and promotes cell-cell adhesion during angiogenesis, playing important roles in the regulation of angiogenic sprouting. These studies also suggest that pharmacologic agents that selectively activate PKA in proliferating endothelial cells could be useful tools to inhibit pathologic angiogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by grant nos. CA83133 and CA98048 to J.V. from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: H.J. performed biochemistry and cell biology; B.G.-S. performed in vivo angiogenesis and vascular leak analyses; C.J.A. performed siRNA studies; K.S. and R.L.K. performed zebrafish studies; and J.A.V. directed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The currrent affiliation for H.J. is Department of Pharmacology, Case Western Reserve University, Cleveland, OH. The current affiliation for B.G.-S. is L'Institut de Médecine Moléculaire de Rangueil à Toulouse, Toulouse, France.
Correspondence: Judith A. Varner, Moores UCSD Cancer Center, 3855 Health Sciences Dr, La Jolla, CA 92093-0819; e-mail: jvarner@ucsd.edu.

![Figure 1. PKA activation suppresses angiogenesis. (A) Mean vessel branchpoints ± SEM in CAMs of 10-day-old chicken embryos stimulated with saline or bFGF and topically treated with saline, 20μM PTHrP, calcitonin gene related peptide (CGRP), or isoproterenol, n = 10, *P < .05. (B) Images of CAMs stimulated with saline or bFGF and treated with saline, PTHrP, or isoproterenol. (C) Mean vessel branchpoints ± SEM in chorioallantoic membranes of 10-day–old chicken embryos stimulated with saline or bFGF, transfected with N1-GFP or pcDNA 3.1 V5/his dnPKA (dominant negative PKA = mutationally inactive PKA regulatory subunit I) as previously described10 and topically treated with saline or PTHrP, n = 10, *P < .05. (D) Mean vessel branchpoints ± SEM in chorioallantoic membranes of 10-day-old chicken embryos stimulated with saline, VEGF-A or bFGF, and topically treated with dibutyryl cAMP or saline, n = 10, *P < .05. (E) Images of 32 hpf transgenic GFP-expressing zebrafish larvae in vasculature [TG(fli1:EGFP1)] after treatment for 24 hours with forskolin or H89, a PKA inhibitor. DA (dorsal aorta), PCV (posterior cardinal vein), ISVs (intersegmental vessels), and DLAVs (dorsal longitudinal anastomotic vessels) are indicated for control-treated embryos.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/25/10.1182_blood-2010-07-296210/4/m_zh89991061940001.jpeg?Expires=1769127050&Signature=erXNfU3fKPpoOmqGl1CYtcrgnAc2sfWZwCfuOMi~odOy2Yjcs0GuWXWTy9WjLEZlmtmi-0vwfbA7M53sL7wpI-rwXEOQG4JzrYeZ~jRbHqpZOUeYBsEfbg3njsT5JMvmBb8Pjdnc25yJoHYwBN6qpI0rYXG8dMyq6V77z3QixOdbyRisxKMsXvdL3UjN30AezalkHcF~gW6xq9KRtRXgjFtoN9DW7AdPGUX3HNVUwllDkcXg5ANXxwcMAV3J6C3F305NRiPgFI0fC8zTr2qRe5IGGWNo-DyA0MmJkI4M--SWlnstu-PftwypAmjyK18I~z5dq02k~~blT1Al~zj87Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 1. PKA activation suppresses angiogenesis. (A) Mean vessel branchpoints ± SEM in CAMs of 10-day-old chicken embryos stimulated with saline or bFGF and topically treated with saline, 20μM PTHrP, calcitonin gene related peptide (CGRP), or isoproterenol, n = 10, *P < .05. (B) Images of CAMs stimulated with saline or bFGF and treated with saline, PTHrP, or isoproterenol. (C) Mean vessel branchpoints ± SEM in chorioallantoic membranes of 10-day–old chicken embryos stimulated with saline or bFGF, transfected with N1-GFP or pcDNA 3.1 V5/his dnPKA (dominant negative PKA = mutationally inactive PKA regulatory subunit I) as previously described10 and topically treated with saline or PTHrP, n = 10, *P < .05. (D) Mean vessel branchpoints ± SEM in chorioallantoic membranes of 10-day-old chicken embryos stimulated with saline, VEGF-A or bFGF, and topically treated with dibutyryl cAMP or saline, n = 10, *P < .05. (E) Images of 32 hpf transgenic GFP-expressing zebrafish larvae in vasculature [TG(fli1:EGFP1)] after treatment for 24 hours with forskolin or H89, a PKA inhibitor. DA (dorsal aorta), PCV (posterior cardinal vein), ISVs (intersegmental vessels), and DLAVs (dorsal longitudinal anastomotic vessels) are indicated for control-treated embryos.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/25/10.1182_blood-2010-07-296210/4/m_zh89991061940001.jpeg?Expires=1769127051&Signature=ViycVX9toVSPqMzzgTiZhXf7bPUkgKcShMcfJ0A-x5JYLO45IOTW5LV4~hrBRKu2WnXq3eHHw5o7ZkAwUNqkbmOjrKMS~OiqZx6APZO8dypVSW8pgVwUOXKJZuyvMrPSSKehWGLysuM0EJ7Fk8M24ZKeffjysoZC073B0Ntvjbe3qiKBVUuYwoVxpfl2DyYtX5scoQU2H97ANdkB-v2RRBCOfr-TeKBLjJCwntzJC6ExzUgBCJEi9jN~MkcPeiHpudWldKv~MqfFlrOb3cClQo9HSRh7g0VGdt3HJOyPz~uwZw8sX~mPrLMzFIoFuAXjvdjg6N8NpRTYj0B5z2tm5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)