This retrospective study assessed the outcome of 576 adult acute lymphoblastic leukemia patients aged ≥ 45 years, and who received a reduced-intensity conditioning (RIC; n = 127) or myeloablative conditioning (MAC; n = 449) allogeneic stem cell transplantation (allo-SCT) from a human leukocyte antigen-identical sibling while in complete remission. With a median follow-up of 16 months, at 2 years, the cumulative incidences of nonrelapse mortality and relapse incidence were 29% ± 2% (MAC) versus 21% ± 5% (RIC; P = .03), and 31% ± 2% (MAC) versus 47% ± 5% (RIC; P < .001), respectively. In a multivariate analysis, nonrelapse mortality was decreased in RIC recipients (P = .0001, hazard ratio [HR] = 1.98) whereas it was associated with higher relapse rate (P = .03, HR = 0.59). At 2 years, LFS was 38% ± 3% (MAC) versus 32% ± 6% (RIC; P = .07). In multivariate analysis, the type of conditioning regimen (RIC vs. MAC) was not significantly associated with leukemia-free survival (P = .23, HR = 0.84). Despite the need for randomized trials, we conclude that RIC allo-SCT from a human leukocyte antigen-identical donor is a potential therapeutic option for acute lymphoblastic leukemia patients aged ≥ 45 years in complete remission and not eligible for MAC allo-SCT.
Introduction
Acute lymphoblastic leukemia (ALL) accounts for approximately 15% to 20% of all adult acute leukemias.1 Although 80% to 90% of adult patients with ALL succeed in achieving complete remission (CR), most of them will relapse and die of their disease.2 Among adults with ALL, long-term leukemia-free survival (LFS) rates of 30% to 40% have been obtained with the use of chemotherapy, compared with 45% to 75% with the use of conventional myeloablative conditioning (MAC) allogeneic stem cell transplantation (allo-SCT).3,,,–7 The latter favorable effect is likely due to a reduced risk of relapse, especially in patients in first CR. However, nonrelapse mortality (NRM) may counterbalance that favorable overall outcome observed after MAC allo-SCT in elderly patients and patients with comorbidities. Thus, the use of reduced intensity conditioning (RIC) before allo-SCT may offer hitherto unavailable opportunities to obtain a graft-versus-leukemia effect without the toxicities of intense preparative regimens. We report herein a retrospective comparative study which assessed the outcomes of 576 adult (age at transplantation ≥ 45 years) patients with ALL who underwent allo-SCT in CR with a human leukocyte antigen (HLA)–identical sibling donor, and analyzed according to the type of conditioning received before allo-SCT (RIC vs MAC).
Methods
Study design and data collection
This was a retrospective multicenter analysis. Data of adult ALL patients receiving RIC or MAC allo-SCT were provided by the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) group. EBMT registry is a voluntary working group of more than 450 transplant centers, participants of which are required once a year to report all consecutive stem cell transplantations and follow-up. The Acute Leukemia Working Party of the EBMT group approved this study.
Criteria of selection
The study included ALL patients receiving first RIC or MAC allo-SCT in first or second CR from an HLA-identical related donor, who (1) were aged ≥ 45 years at time of transplant; (2) were transplanted between 1997 and 2007; (3) had received a MAC regimen (standard high-dose radio- or chemotherapy) or a RIC regimen defined as the use of fludarabine associated with low-dose total body irradiation (TBI; ≤ 6 Gy) or busulfan (total dose ≤ 8 mg/kg), or other immunosuppressive or chemotherapeutic drugs such as melphalan or cyclophosphamide8 ; or (4) were patients whose clinical data on outcomes were adequate. A total of 576 allo-SCT recipients from 186 transplant centers met these eligibility criteria.
Patients and transplant procedures
Differences between patients, disease, and transplant-related factors according to conditioning regimen are given in Table 1. As per EBMT centers practice for allo-SCT in ALL, patients were eligible to receive a MAC regimen if they were aged under 50 or 55 years and did not have significant comorbidities that precluded the use of high-dose radio- or chemotherapy (n = 362). In addition, 87 patients who were aged > 55 years received a MAC regimen because they did not have significant comorbidities and were judged as “fit” to receive high-dose therapy. In the RIC group, 110 patients (87%) received a RIC regimen mainly because of age ≥ 50 years irrespective of the presence or absence of significant comorbidities. The remaining 17 patients (13%) who received a RIC regimen were aged < 50 years but had one or more comorbidities or specific reasons that precluded the use of high-dose therapy (see details in Table 1). The majority of the patients in the RIC group (91%) received peripheral blood stem cells compared with 66% in the MAC group. For graft-versus-host disease (GVHD) prevention, 81% of the patients in the MAC group received the classical cyclosporine A (CsA) plus methotrexate combination, while patients in the RIC group received either, CsA alone (27%), CsA plus mycophenolate mofetil (36%) or CsA plus methotrexate (37%). Four hundred ninety-six (86%) patients were transplanted in first CR, while the remaining patients were transplanted in second CR (n = 80). The median follow-up was 16 months (range, 1-119) months.
Statistical analysis
The probabilities of overall survival (OS), LFS, relapse incidence (RI), and NRM were the primary study end points. LFS was defined as survival without evidence of relapse or progression. LFS and OS were calculated using the Kaplan-Meier estimate. RI, NRM, as well as the probabilities of acute and chronic GVHD were calculated using cumulative incidence (CI) in a competing risks setting, with death in remission treated as a competing event to relapse.9 Univariate analyses were done with the use of log-rank test for OS and LFS while the Gray test was applied for RI and NRM. Patient-, disease-, and transplant-related variables of the 2 groups were compared, using the χ2 statistic for categorical and the Mann-Whitney test for continuous variables. Multivariate analyses were performed using Cox proportional hazards model including chronic GVDH as a time-dependent variable. Factors differing in term of distribution between the 2 groups and factors associated with a P value less than < .10 by univariate analysis were included in the final model. For all prognostic analyses, continuous variables were categorized and the median was used as a cutoff point. All tests are 2-sided. The type I error rate was fixed at 0.05 for determination of factors associated with time to event outcomes. Statistical analyses were performed with SPSS 15.0 (SPSS Inc) and Splus 6.1 (MathSoft Inc) software packages.
Results and discussion
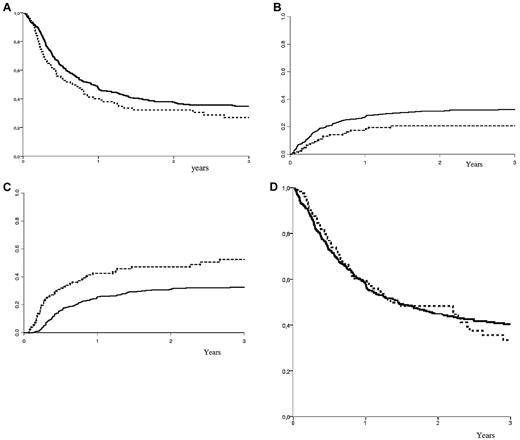
In the total population at 2 years after allo-SCT, OS and LFS were 46% ± 2% and 37% ± 2% respectively. The CIs of grade 2-4 acute and chronic GVHD were 26% ± 3% and 34% ± 3%, respectively, comparable between both MAC and RIC groups (P = .16 and P = .37, respectively). The overall RI and NRM were 34% ± 3%, and 29% ± 3%, respectively. LFS, NRM, RI, and OS according to the type of conditioning regimen are shown in Figure 1. At 2 years, the nonadjusted CI of NRM was 31% ± 2% (MAC) versus 21% ± 4% (RIC; P = .03). The nonadjusted CI of RI at 2 years was 31% ± 2% (MAC) versus 47% ± 5% (RIC; P < .001). At 2 years, there was a trend toward improved LFS in the MAC group: 38% ± 3% (MAC) versus 32% ± 5% (RIC; P = .07). However, OS was similar: 45% ± 3% (MAC) versus 48% ± 5% (RIC; P = .56). In the Philadelphia-positive subgroup of patients (n = 145), the results were 47% ± 5% for OS, 33% ± 5% for LFS, 40% ± 5% for RI, and 27% ± 4% for NRM in the MAC group, versus 40% ± 9% for OS, 34% ± 8% for LFS, 49% ± 9% for RI, and 17% ± 7% for NRM in the RIC group. In patients transplanted in CR1, the results were 48% ± 3% for OS, 40% ± 3% for LFS, 28% ± 2% for RI, and 32% ± 2% for NRM in the MAC group versus 51% ± 6% for OS, 35% ± 5% for LFS, 48% ± 5% for RI, and 17% ± 4% for NRM in the RIC group. In patients transplanted in second CR, the results were 24% ± 7% and 33% ± 11% for OS, 18% ± 6% and 20% ± 10% for LFS, 54% ± 7% and 43% ± 12% for RI, and 28% ± 7% and 38% ± 12% for NRM, in the MAC and RIC groups, respectively. In the MAC group, the incidences of NRM were 23% ± 3%, 38% ± 3%, and 46% ± 18 in the 45 to 50 (n = 218), 50 to 60 (n = 214), and > 60 years (n = 17) age categories, respectively (P = .007). In the RIC group, the incidences of NRM were 14% ± 2% (2 events), 22% ± 5%, and 20% ± 8% in the 45 to 50 (n = 16), 50 to 60 (n = 75), and > 60 years (n = 36) age categories (P = not significant [NS]). In the same age categories, LFS and OS at 2 years were 41% ± 4%, 35% ± 4%, 14% ± 12%, and 50% ± 4%, 40% ± 4%, and 41% ± 15% in the MAC group, respectively. Similarly, LFS and OS at 2 years were 65% ± 13% (4 events), 28% ± 6%, 25% ± 8%, and 79% ± 11% (5 events), 48% ± 7%, and 32% ± 9% in the RIC group, respectively (nonadjusted comparisons for LFS and OS between MAC and RIC within the above age categories were all statistically NS). In a multivariate analysis, adjusting for patient-, disease- and transplant related-factors that were different in both groups, RI was increased in RIC recipients (P = .0001, HR = 1.98); however the use of RIC was associated with lower NRM (P = .03, HR = 0.59). Other factors associated with increased relapse were absence of chronic GVHD and patients transplanted in second CR, whereas other factors associated with decreased NRM were patients younger than 50 years old and male donor. In multivariate analysis for OS, type of conditioning was not associated with OS (RIC vs. MAC: P = .23, HR = 1.21); other factors such as elderly patients (age > 50 years: P = .008, HR = 0.69), disease status at transplant (second CR: P = .001, HR = 0.59), and a female donor to a male recipient (P = .005, HR = 0.68) were factors associated with a decreased OS. In multivariate analysis for LFS, type of conditioning was not associated with LFS (RIC vs MAC: P = .23, HR = 0.84); other factors such as absence of chronic GVHD (P = .01, HR = 0.62), elderly patients (age > 50 years: P = .016, HR = 0.75), disease status at transplant (second CR: P = .004, HR = 0.64), and a female donor to a male recipient (P = .045, HR = 0.77) were factors associated with a decreased LFS.
Survival probabilities. (A) LFS according to conditioning regimen; (B) NRM according to conditioning regimen; (C) relapse incidence according to conditioning regimen; (D) OS according to conditioning regimen. MAC: plain curve; RIC: dashed curve. x-axis: years after transplantation; y-axis: percent outcome.
Survival probabilities. (A) LFS according to conditioning regimen; (B) NRM according to conditioning regimen; (C) relapse incidence according to conditioning regimen; (D) OS according to conditioning regimen. MAC: plain curve; RIC: dashed curve. x-axis: years after transplantation; y-axis: percent outcome.
The role of allo-SCT in adult ALL is still controversial.4,10,–12 In addition, ALL encompasses a group of chemosensitive diseases, raising concerns that significant reduction of the intensity of the preparative regimen before allo-SCT may have a negative impact on long-term leukemic control as it has been already shown in other settings.13,14 Very few data are available analyzing RIC allo-SCT for patients with ALL.15,,–18 Results of this registry analysis suggest that RIC regimens may reduce NRM rate after allo-SCT for adult ALL compared with standard MAC regimens, but with a higher risk of disease relapse and no statistically significant impact on LFS. In another word, there is an apparent trade off, including lower NRM with RIC, but a higher rate of relapse. It is also notable that the RIC patients did not do significantly worse than the MAC patients, although LFS tended to be lower. However, OS, likely the most important outcome for an individual patient, was strictly comparable between both groups.
In the MAC regimens groups (high-dose TBI versus high-dose chemotherapy alone), LFS, NRM, and relapse incidence were: 40 ± 3 versus 28 ± 6 (P = .06), 31 ± 3 versus 30 ± 5 (P = .98), and 28 ± 3 versus 42 ± 6 (P = .04), respectively. On the other hand, although RIC regimens were heterogeneous, there were no statistically significant differences in NRM and relapse incidences for the different RIC categories (NRM: 18% ± 8%, 23% ± 9%, and 23% ± 9% [P = NS]; relapse: 48% ± 9%, 55% ± 11%, and 45% ± 12% [P = NS] in the low-dose TBI-based RIC, fludarabine-busulfan, and fludarabine-melphalan RIC regimens, respectively).
One major limitation in this type of analysis is that the patient populations are fundamentally different. Patients received a MAC regimen unless they were elderly or had major comorbidities. Thus, the RIC patients were older (6 years older) or had comorbidities precluding the use of MAC. Thus, the most relevant question would be whether RIC in older patients produces an acceptable outcome. However, despite the latter limitations, this analysis contains some promising findings, since it was focused on patients aged ≥ 45. Indeed, those patients who received RIC are likely to have serious comorbidities, which led the transplantation center to choose RIC instead of MAC.19 In fact, age older than 45 to 50 years is widely accepted as a major prognostic variable for increased NRM after allo-SCT.20 In addition, toxicity of the intensive chemotherapy used in ALL to achieve initial or subsequent remission before transplant can compromise a patient's ability to tolerate a MAC regimen. Unfortunately, it was not possible to provide the details of the prior chemotherapy used in these patients and assess the impact of this parameter on outcome. Obviously, any comparison between RIC and MAC may be influenced by a selection bias. However, to adjust for differences between patients selected for RIC or MAC, we had enough patients in this study to perform a multivariate analysis and adjust for differences between the 2 groups. Moreover, we focused on those patients in CR and who received allo-SCT from an HLA-identical donor. With these considerations in mind, these results are encouraging in this subgroup of ALL patients aged ≥ 45, especially in term of OS. Moreover, in the RIC group, LFS in patients aged ≥ 60 years was 25% ± 8% at 2 years comparing favorably with results of chemotherapy alone in elderly ALL,1 and further highlighting the overall benefit of RIC allo-SCT in elderly ALL.18 However one should bear in mind that the value of allo-SCT versus chemotherapy is definitely among the key issues that need to be further assessed. Indeed, given the increasingly improved results and safety of chemotherapy alone, the comparison of transplant versus chemotherapy at different age groups warrants some well designed and rigorous prospective trials because the current study suggests that the results of the RIC allo-SCT approach look promising versus chemotherapy alternatives for those ALL patients aged > 45 years.
The use of preemptive donor leukocyte infusion immediately after RIC allo-SCT may be also expected to be beneficial, especially in patients with a positive residual disease,21 but this could not be assessed in this cohort. In addition, amplification of the graft-versus-leukemia effect may be one way to reduce the rate of relapse.22 Post-RIC allo-SCT maintenance therapy (eg, tyrosine kinase inhibitors) may also succeed in eradicating residual leukemic cells.23 Monoclonal antibodies directed against antigens expressed by leukemic cells (anti-CD20/22/33) may be also less toxic and more efficient than chemotherapy.24 Despite the need for prospective randomized studies with analyses based on the intention–to–treat principle, we conclude that RIC allo-SCT from an HLA-identical donor is a feasible and potential therapeutic option for ALL patients aged ≥ 45 in CR and not eligible for MAC allo-SCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank E. Polge and B. Samey from the office of the Acute Leukemia Working Party of EBMT.
Authorship
Contribution: M.M., M.L., and V.R. conceived and designed the study, provided administrative support, performed data analysis and interpretation, and revised the manuscript; M.M. and V.R. arranged financial support; M.M., L.V., A.G., G.S., J.E., R.T., A.N., and V.R. provided study materials or patients; M.L. collected and assembled data and conducted statistical analyses; M.M. wrote the manuscript; and M.M., M.L., L.V., A.G., G.S., J.E., R.T., A.N., and V.R provided final manuscript approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mohamad Mohty, Service d'Hématologie Clinique, CHU Hôtel-Dieu, Université de Nantes and Inserm U892, Place Alexis Ricordeau, F-44093 Nantes, France; e-mail: mohamad.mohty@univ-nantes.fr.