Abstract
Abstract 5041
Novel biological agents, particularly bortezomib (B) and the immunomodulatory agents thalidomide (T) and lenalidomide (L), have improved the response rates and the survival in multiple myeloma (MM). However, patients refractory to, or progressing after, treatment with one or more new agents have a very poor prognosis. Alkylating agents are often avoided in the initial management of MM patients who are eligible for autologous hematopoietic stem cell (HSC) transplantation due to concerns for impairment of HSC mobilization. We hypothesized that high doses of cyclophosphamide (HiCy) administered without HSC support may be an effective and safe treatment to rescue patients with relapsed or refractory MM previously treated with novel biological agents.
We performed a retrospective single institution review of all recent patients receiving high dose cyclophosphamide (3000mg/m2) after failure of one or more new biological agents. Data extracted included patient demographics, disease characteristics, treatment history, toxicity, response, and survival data. This study received institutional review board approval.
Between 03/2009 and 06/2010, 11 patients were treated with cyclophosphamide 3,000mg/m2 for relapsed or refractory MM. Supportive care included administration of mesna to prevent hemorrhagic cystitis and PEG-filgrastim to minimize the duration and depth of neutropenia. All patients received antibiotic prophylaxis with ciprofloxacin, acyclovir, and fluconazole. The median age of patients was 52 years (range 26–73). Among these, 7 patients (64%) had stage 2 (international staging system), and 4 patients (36%) had stage 3 disease. Metaphase cytogenetics and/or fluorescence in situ hybridization (FISH) was available for 10 patients. In all but one patient, MM cells had FISH abnormalities. Cytogenetic subgroups included five cases with complex karyotype, 6 cases with a deletion of chromosome 13, 3 cases with abnormalities involving 17p, 2 cases with t(11;14), 2 hyperdiploid cases, and 1 case with t(14;16). The median number of prior treatments was 3 (range 1–6) with two patients previously receiving autologous HSC transplantation. All patients were refractory to the last therapeutic regimen and had failed to respond or were refractory to a regimen containing B. Seven patients had also previously received an immunomodulatory agent (2 patients received T and 6 patients received L). At the time of treatment 5 patients had plasma cell leukemia and 5 patients had renal failure (including 2 patients on dialysis). All patients developed grade 4 neutropenia, and 9 patients developed thrombocytopenia requiring transfusion support. Six patients (55%) developed fever and neutropenia requiring intravenous antibiotics, and one patient (9%) had documented bacteremia. One patient died from sepsis 7 weeks after cyclophosphamide treatment. Median follow up was 13.4 weeks (range 4.4–69.7) from HiCy administration. Overall, 7 patients had a partial response or better (64%), including 3 patients with a very good partial response (27%). Four of the 7 responding patients subsequently underwent autologous (3) or allogeneic (1) HSC transplantation. Median overall survival was not reached, and the estimated one year survival was 52.5% (+/− 20.4%).
High-dose cyclophosphamide can be an effective salvage therapy for young, high-risk MM patients refractory to new biological agents. HiCy is associated with significant toxicity, and careful patient selection is necessary.
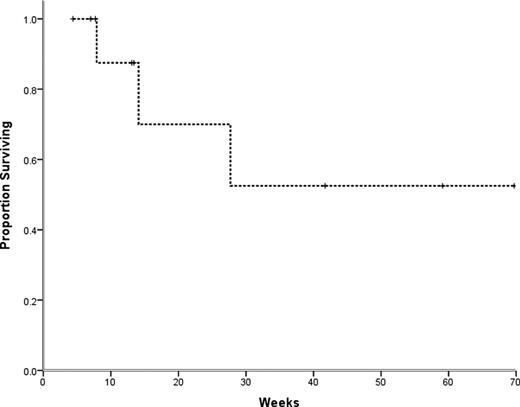
Proportion of refractory multiple myeloma patients surviving after treatment with high-dose cyclophosphamide over time (wks). Median overall survival was not reached, and the estimated one year survival was 52.5% (SE 20.4%).
Proportion of refractory multiple myeloma patients surviving after treatment with high-dose cyclophosphamide over time (wks). Median overall survival was not reached, and the estimated one year survival was 52.5% (SE 20.4%).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.