Abstract
Abstract 4033
DNMTIs are the most commonly used disease modifying agents for myelodysplastic syndromes (MDS), particularly in patients (pts) with higher-risk MDS. Many will subsequently develop acute myeloid leukemia (AML) and will be treated with cytarabine-based remission induction therapy. The clinical impact of prior DNMTI therapy on the effectiveness of AML induction therapy is unknown for pts with AML arising secondary to MDS (sAML).
We reviewed all pts treated with cytarabine-based induction chemotherapy for sAML arising from MDS at Cleveland Clinic between 2002 and 2009, years during which DNMTI therapy may have been used for MDS. Data on demographics, cytogenetic risk classification (per CALGB 8461), and disease duration were collected for MDS and for AML diagnoses. Response to DNMTI therapy (using International Working Group (IWG) 2006 criteria), complete response (CR) to induction chemotherapy (using IWG 2003 AML criteria), and overall survival (OS) were analyzed; comparisons between groups were performed using univariate and regression analyses, controlling for age, gender, white blood cell count, cytogenetics, reinduction, disease duration, and International Prognostic Scoring System (IPSS) score.
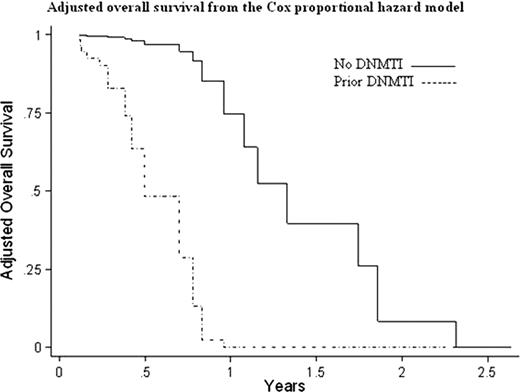
Of 330 AML pts treated with remission induction chemotherapy over the 7-year period, 27 had an antecedent MDS; 14 were treated with DNMTI prior to AML transformation, 13 were not. For these sAML pts, mean age was 67.5 years (range, 39–85), 11 (41%) were female, AML cytogenetic risk groups were intermediate (13), poor (10), and unknown (4), while IPSS cytogenetic risk groups were good (12), intermediate (7), poor (3), and unknown (5). Only 2 pts (7%) had a chromosome 7 abnormality. By MDS WHO classification, pts had RA/RARS (4), RCMD (6), RAEB-1 (6), RAEB-2 (9), and CMML-1 (2). Pts had MDS a median of 1.76 years (range .33-6.0). Compared to pts not treated with DNMTI, pts who received a DNMTI for MDS were younger (63 vs. 72 years, p=.013) but duration from MDS diagnosis to AML diagnosis was similar between groups (1.6 years vs 1.9 years, p=.60), as were other baseline factors identified above. Among sAML pts, reinduction rate for persistent leukemia was qualitatively higher in the DNMTI group (36% vs 8%), with a trend towards significance (p=.08). CR to AML induction for the sAML pts was 41% (compared to 52% for all AML patients ≥60 years who were induced), but was significantly worse in the DNMTI group (21% vs 62% for non-DNMTI, p=.034), and by log rank there was a trend toward worse OS in those treated with a DNMTI (.5 years vs 1.1 years, p=.11, similar to all AML patients ≥60 years, at .41 years). When compared to sAML without prior DNMTI, by logistic regression analyses, prior use of a DNMTI led to a worse CR rate to induction (hazard ratio .026, p=.047), and using Cox regression, prior use of a DNMTI led to worse OS (hazard ratio 22.95, p=.004). Within the MDS group treated with a DNMTI, achievement of complete or partial response by IWG MDS criteria was significantly correlated with CR to induction chemotherapy for AML (Pearson correlation coefficient .61, p=.021) but not with OS (Pearson correlation coefficient .40, p=.15).
Induction therapy in sAML pts previously treated with DNMTI for MDS is less effective and associated with worse OS when compared to sAML pts not treated with DNMTI, controlling for baseline factors including MDS duration and IPSS score. However, within this poor-risk group, clinical response to DNMTI therapy is correlated with improved CR to AML induction therapy. Treatment alternatives to induction chemotherapy for AML after prior DNMTI therapy should be investigated.
Mohan:Celgene: Research Funding; Eisai: Research Funding. Maciejewski:Celgene: Research Funding; Eisai: Research Funding. Sekeres:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.