Abstract
Abstract 3501
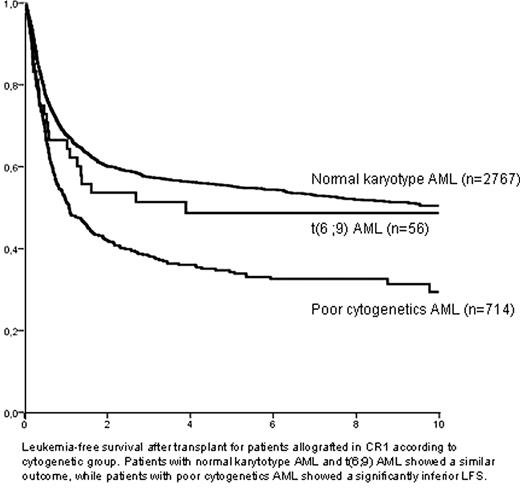
Acute myeloid leukemia (AML) with translocation t(6;9)(p23;q34)/DEK-NUP214(CAN) rearrangement (t(6;9) AML) is a rare but well-characterized entity, associated to a poor prognosis. In this regard, a possible benefit of allogeneic hematopoietic stem-cell transplantation (alloHSCT) has been suggested, based on small series of patients. To investigate the potential role of alloHSCT for the management of t(6;9) AML we analyzed the outcome of patients with this AML subtype submitted to alloHSCT and reported to the ALWP, and compared it to other well-defined cytogenetic categories. Overall, we identified 74 patients (median age: 38, 18–65; 51% male) diagnosed with t(6;9) AML allografted since 1988 (median year of transplant: 2004). Most transplants were performed in complete response (CR1=56, 76%; CR2=8, 11%), whereas only a minority were performed in advanced phase (primary refractory, n=5; relapse, n=5). Donor was an HLA-identical sibling in 43 transplants (58%), and a matched unrelated donor in 24 (32%). Conditioning regimen consisted of a myeloablative regimen in most patients (n=61, 82%), and source of stem-cells was peripheral blood in 41 (55%) and bone marrow in 32 (43%). After a median follow-up of 51 months, 3-year leukemia-free survival (LFS), relapse incidence (RI), and non-relapse mortality (NRM) for patients allografted in CR1 was 51±7%, 19±6%, and 30±7%, respectively, whereas LFS for patients transplanted in other disease status was only 16±10% (p<0.0001). A multivariate analysis performed among patients who received alloHSCT in CR1 identified a short interval CR-alloHSCT (<90 days) as the only favorable outcome for LFS (3-yr LFS: 57±10% vs. 51±7%; hazard ratio, HR=0.36, 95% CI:0.15-0.89; p=0.03) and NRM (47±11% vs. 17±8%; HR:3.84, 1.18–12.5; p=0.03), whereas reduced-intensity conditioning was followed by a higher RI (3-yr RI: 32±20% vs.17±6%; HR:4.86, 1.06–22.36; p=0.04). Moreover, the outcome of t(6;9) AML patients submitted to alloHSCT in CR1 was compared to that of patients with normal cytogenetics AML (NC-AML, n=2767) and poor cytogenetics AML (PR-AML, n=714) also allografted in CR1 in a multivariate analysis which included main prognostic variables. Interestingly, LFS and RI after alloHSCT of t(6;9) AML patients was similar to that observed in patients with NC-AML (51±7% and 58±1% for LFS, 19±7% and 23±1% for RI, respectively). On the contrary, the outcome of PR-AML was significantly poorer to NC-AML, with a 3-yr LFS and RI of 38±2% (p<0.0001; HR=1.58, 1.39–1.82) and 41±2%, respectively (p<0.0001; HR=2.09, 1.76–2.49; figure). In conclusion, alloHSCT in early phase resulted in a favourable outcome in patients with AML associated to translocation t(6;9), comparable to that of patients with NC-AML, suggesting that this procedure might overcome the adverse prognosis associated to this entity.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.