Abstract
Abstract 2172
AML patients with FLT3/ITD mutations have an inferior survival compared to AML patients with wild-type (WT) FLT3, primarily due to an increased relapse rate. Allogeneic transplant represents a post-remission therapy that is effective at reducing the risk of relapse for many cases of poor-risk AML. Whether or not allogeneic transplant in first complete remission (CR) can improve outcomes for patients with FLT3/ITD AML remains controversial. Our institution has adopted a policy of pursuing allogeneic transplant, including the use of alternate donors, for FLT3/ITD AML patients in remission.
As part of an IRB-approved study, we performed a review of the clinical data from November 1, 2004 to October 31, 2008 on all adult patients under the age of 60 presenting in consecutive fashion to the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins with newly diagnosed non-M3 AML. We followed their outcomes through August 1, 2010.
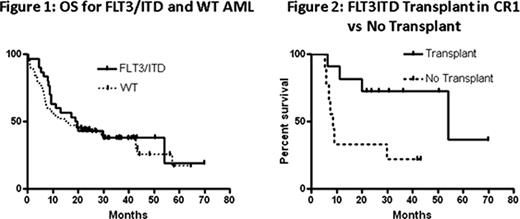
During the study period, 133 previously untreated AML patients between the ages of 20 and 59 were diagnosed and received induction and consolidation therapy at our institution. Of these 133 patients, 31 (23%) harbored a FLT3/ITD mutation at diagnosis. Fourteen of the total 133 patients were deemed unfit for intensive therapy, all WT patients. Of these, 6 (5%) received investigational agents on protocol while the other 8 (6%) were treated with combinations of supportive care and cytotoxic agents off protocol. Of the remaining patients, 119/133 (89%) received timed- sequential intensive induction therapy (TST) with infusional cytarabine, an anthracycline or anthracenedione, and a third drug. The majority, 95/133 (71%), received the three drug TST regimen of cytarabine, daunorubicin, and etoposide delivered in a timed sequential fashion, while 24/133 (18%) were treated with TST containing cytarabine, mitoxantrone, and the investigational agent flavopiridol on an institutional protocol. 72/119 (61%) achieved a CR with induction therapy (55 patients in AcDVP16 group and 17 patients in FLAM group). The OS (overall survival) from the time of diagnosis for the FLT3/ITD AML patients was compared to the OS of the entire cohort (n=133) (Figure 1). The median OS for the FLT3/ITD group was 19.3 (range 0.0–69.9) months and the median OS for the WT patients was 15.5 (range 0.7 to 64.6) months (p=0.56). Of the FLT3/ITD patients, 20/31 (65%) achieved a complete remission with intensive induction therapy. Of these 20 patients in CR1, 11 (55%) underwent an allogeneic transplant in first remission (4 HLA-matched myeloablative sibling, 5 HLA-matched myeloablative unrelated donor, and 2 nonmyeloablative haplo-identical related donor) compared to only 18/102 (17%) WT patients were transplanted in first CR, a statistically different proportion compared to the FLT3/ITD patients (p<0.0001). The 9 FLT3/ITD patients who were not transplanted in CR1 had issues related to lack of donor or overall comorbidities. There was one patient who went on to transplant after first relapse. The median relapse-free survival in the FLT3/ITD non- transplant group was 8.6 (range 5.3–43.3) months while the median relapse-free survival in the FLT3/ITD transplant group was 54.1 (range 6.4–69.9) months (p=0.02) (Figure 2).
Historically, OS for FLT3/ITD AML patients is significantly worse than for AML patients lacking this mutation. However, the OS for the 31 FLT3/ITD patients reported here was comparable to the 102 patients with WT FLT3 over the same 4 year time period. One difference that might have contributed to the surprising outcomes for the FLT3/ITD group is our aggressive pursuit of allogeneic BMT in CR1 within this group (60% of FLT3/ITD vs 17% with WT). Our single institution study of consecutively treated AML patients supports the hypothesis that allogeneic transplant in early CR1 improves the long term outcomes for FLT3/ITD AML.
Levis: Ambit Biosciences, Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.