Abstract
Abstract 1779
Tumor Lysis Syndrome (TLS) is an oncologic emergency that leads to a host of metabolic disturbances which can ultimately result in acute renal failure and death.
Currently rasburicase, Elitek™, is FDA approved for the treatment of TLS in patients with leukemia, lymphoma, and solid tumor malignancies which have risk factors for TLS. The approved adult dose is 0.2mg/kg/day over 30 minutes for up to 5 days. Chemotherapy should be initiated within 24 hours of rasburicase administration. Since rasburicase's introduction to the market, various studies have examined single dose use (3mg and 6mg) in the adult population, trying to resolve the dilemma of determining a dosing regimen for adults that is as effective as the FDA approved dosing regimen, while minimizing cost.
This study was aimed at determining if a single 4.5mg dose of rasburicase would be adequate in reducing uric acid levels (UAL) as compared to the FDA approved conventional weight based approach; it also sought to determine the cost-effectiveness of this approach.
This is a retrospective study of the John H. Stroger Jr. Hospital of Cook County (JHS) patients who were administered a single dose of 4.5 mg rasburicase for TLS from 12/2007 to 06/2010. We included patients ≥ 18 years old, with a hematologic malignancy and on chemotherapy or about to start receiving chemotherapy within 24 hours of rasburicase administration. Patients with low risk for TLS or an indication other than prevention and management of TLS were excluded.
Demographics:
| Total number of patients: | 25 |
| Male 18 | (72%) |
| Female | 7 (28%) |
| Average age (years) | 54 |
| Average weight (kg) | 78.1 |
| Race | |
| African American | 15 (60%) |
| White | 6 (24%) |
| Hispanic | 3 (12%) |
| Asian | 1 (4%) |
| Total number of patients: | 25 |
| Male 18 | (72%) |
| Female | 7 (28%) |
| Average age (years) | 54 |
| Average weight (kg) | 78.1 |
| Race | |
| African American | 15 (60%) |
| White | 6 (24%) |
| Hispanic | 3 (12%) |
| Asian | 1 (4%) |
Characteristics:
| Number of events | 30 |
| Number of doses administered | 33 |
| Number of patients with hyperuricemia | 24 (96%) |
| TLS Grade* (number of events) | |
| 0–1 | 14 |
| 2–3 | 16 |
| 4–5 | 0 |
| Malignancy types (N=25 patients) | |
| Leukemia | 7 (28%) |
| Lymphoma | 13 (52%) |
| Myeloma | 5 (20%) |
| Responders (based on events) | 28 |
| Non-responders | 2 |
| Number of events | 30 |
| Number of doses administered | 33 |
| Number of patients with hyperuricemia | 24 (96%) |
| TLS Grade* (number of events) | |
| 0–1 | 14 |
| 2–3 | 16 |
| 4–5 | 0 |
| Malignancy types (N=25 patients) | |
| Leukemia | 7 (28%) |
| Lymphoma | 13 (52%) |
| Myeloma | 5 (20%) |
| Responders (based on events) | 28 |
| Non-responders | 2 |
Cairo. Br J Haematol. 2004;127:3.
Responders are defined as patients that achieved more than 50% reduction in the UAL at 24 hours, 48 hours or 96 hours or a decrease of UAL to normal levels. Two patients required a second dose to achieve response. The non-responders did not receive any additional doses for unclear reasons. No adverse events were noted.
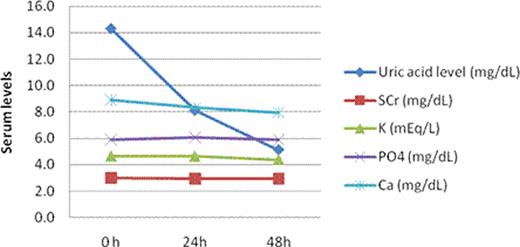
This retrospective study provides evidence that a single 4.5mg dose of rasburicase effectively reduced plasma UAL to within normal limits. In addition, decrease in plasma UAL was observed within 24 hours after administration. No effect on electrolytes and serum creatinine was noted.
The cost-effectiveness of a single 4.5mg dose of rasburicase was evaluated based on dose and drug cost. The potential cost saving was substantial when compared with the FDA approved dose.
| Dosing . | Cost per patient* . | Total cost (for 30 events) . | Total savings (4.5mg vs. FDA approved regimen) . | |
|---|---|---|---|---|
| Single 4.5mg dose regimen | $1,919.31 | $53,548.75 | $906,106.25 | |
| FDA approved 5 day regimen | $31,988.5 | $959,655 | ||
| Dosing . | Cost per patient* . | Total cost (for 30 events) . | Total savings (4.5mg vs. FDA approved regimen) . | |
|---|---|---|---|---|
| Single 4.5mg dose regimen | $1,919.31 | $53,548.75 | $906,106.25 | |
| FDA approved 5 day regimen | $31,988.5 | $959,655 | ||
Cost was calculated using August 2010 AmeriSource-Bergen Bluebook Average Wholesale Price. One 1.5mg vial of rasburicase costs $639.77; therefore for a 75kg patient dosed at 0.2mg/kg, the price for one dose would be approximately $6,397.7 and $31,988.5 for a 5 day therapy.
In our opinion, this study validates the use of a single dose of 4.5mg rasburicase in the treatment and prophylaxis of TLS. This dose can be considered as a possible alternative to the FDA approved adult dosing regimen.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.