Abstract
Abstract 1518
Haemophilia A is a rare inherited bleeding disorder characterised by spontaneous or trauma-related bleeding, most commonly into the joints, leading to pain, swelling and limited movement. Some 30–50% of patients with haemophilia A develop inhibitors to their standard treatment, comprised of FVIII concentrates. There are two bypassing agents used for the management of bleeding episodes in patients with haemophilia A with inhibitors in the UK: recombinant factor VII (rFVIIa, NovoSeven®) and plasma-derived activated prothrombin complex concentrate (pd-aPCC, Feiba®).
A systematic review of the cost-effectiveness of pd-aPCC versus rFVIIa was published in 2003. Since the analysis, costs of bypassing agents in the UK have changed significantly (pd-aPCC +30% from ≤0.57/U to ≤0.74/U; rFVIIa -11% from ≤0.51/mcg to ≤0.46/mcg). The present study examines the evolution in costs of resolving a mild to moderate bleeding episode with treatment initiated outside hospital.
A decision analytic model estimated the expected costs and outcomes of resolving a mild-moderate bleeding episode in patients with haemophilia A with inhibitors. The model compared three treatment regimens in patients with a mean weight 70kg. Each regimen comprised first-, second- and third-line treatment: (1) pd-aPCC-pd-aPCC-rFVIIa; (2) pd-aPCC-rFVIIa-rFVIIa; and (3) rFVIIa-rFVIIa-rFVIIa. Efficacy and dosing of pd-aPCC and rFVIIa were estimated from the published literature, with rFVIIa assumed to resolve 92% of mild-moderate bleeds when used as first and second line treatment and pd-aPCC resolving 79% and 88% of mild-moderate bleeds when used first and second line respectively. Patients whose bleeding episodes were not resolved with first-line treatment were admitted to hospital for second- and third-line treatment. Costs included in the economic model were bypassing agent costs and hospitalisation costs.
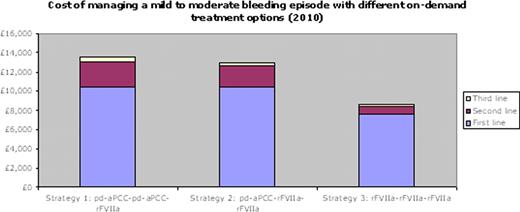
The different treatment regimens result in different total costs for the resolution of a single mild-moderate bleeding episode with up to three lines of treatment: (1) GBP 13,542 (EUR 15,180); (2) GBP 12,985 (EUR 14,556); and (3) GBP 8,569 (EUR 9,605). Since 2001, the costs of regimens 1 and 2 have increased by 26% and 18%, but regimen 3 has decreased by 11% in the UK.
Divergence in the price of pd-aPCC and rFVIIa since 2001 has increased the cost-effectiveness of rFVIIa. The results reinforce the fact that effective first-line treatment with rFVIIa results in lower overall treatment costs due to a reduced need for further treatment and associated hospitalisation costs. Adopting rFVIIa as first-line treatment is cost-saving and more effective compared to reserving rFVIIa as a second- or third-line treatment option.
Sewpaul:Novo Nordisk : Employment. Ashley:Novo Nordisk : Employment. Riley:Novo Nordisk Ltd: Employment.
Author notes
Asterisk with author names denotes non-ASH members.