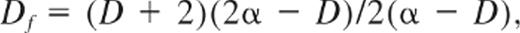Abstract
Here we report the first application of a fractal analysis of the viscoelastic properties of incipient blood clots. We sought to ascertain whether the incipient clot's fractal dimension, Df, could be used as a functional biomarker of hemostasis. The incipient clot is formed at the gel point (GP) of coagulating blood, the GP demarcating a functional change from viscoelastic liquid to a viscoelastic solid. Incipient clots formed in whole healthy blood show a clearly defined value of Df within a narrow range that represents an index of clotting in health, where Df = 1.74 (± 0.07). A significant relationship is found between the incipient clot formation time, TGP, and the activated partial thromboplastin time, whereas the association of Df with the microstructural characteristics of the incipient clot is supported by its significant correlation with fibrinogen. Our study reveals that unfractionated heparin not only prolongs the onset of clot formation but has a significant effect on its fractal microstructure. A progressive increase in unfractionated heparin concentration results in a linear decrease in Df and a corresponding prolongation in TGP. The results represent a new, quantitative measure of clot quality derived from measurements on whole blood samples.
Introduction
Coagulation pathway changes play an important role in the outcome of both clot propagation and fibrinolysis. The structure-function relationship of the developing fibrin clot is known to be affected by many factors, such as environment, therapy, and disease, compared with normal clot growth.1,2 A fibrin clot's primary microstructure consists of a disordered network of entangled, branching fibrin fibers. Thinner fibers are associated with networks that display an increased number of branch points, creating denser, less permeable clots that have a known association with thromboembolic disease.3-6 More open/permeable networks are formed from thicker fibers, the latter displaying a reduced number of branch points for a given amount of fibrinogen and producing a more porous system.7-10 Clots with altered fibrin microstructure exhibit different susceptibility to fibrinolysis,8,10,11 with clot permeability being the rate-limiting factor for the activity of the fibrin network degradation enzyme plasmin. The permeability will aid or hamper the ability of tissue plasmin activator, tPA, and/or urokinase plasmin activator to move through the 3-dimensional fibrin network and activate the zymogen plasminogen to fibrinolytic plasmin. The effect of anticoagulants such as heparin in the therapeutic manipulation of fibrin clot microstructure by thrombin inhibition increases clot permeability/porosity and produces clots with thicker fibers.12,13
The evolution of clot microstructure is associated with significant changes in blood viscoelasticity (a measure of a material's viscous and elastic properties). Viscoelastic properties are among the most sensitive measures of fibrin polymerization and blood clot structure.7,14 In the present study, we focused on the formation of the incipient clot, which provides the microstructural template that determines the future clot morphology,15-17 by measuring the incipient clot's viscoelastic properties with an oscillatory shear technique known as Fourier transform mechanical spectroscopy (FTMS).18-20 This technique provides an accurate determination of the gel point (GP) of coagulating blood and allows the microstructure of the incipient clot to be quantified by fractal analysis, a technique widely used in medicine and biology to characterize nonlinear growth in branching network structures.21 The authors of previous studies of clot structure on the basis of techniques such as scanning electron microscopy have reported qualitative descriptions of clot microstructure (involving terms such as “rigid clot structures,” “open/porous/dense/loose,” etc1 ), whereas studies of the fractal properties of fibrin gels on the basis of light-scattering techniques have been restricted to dilute solutions of fibrinogen, at concentrations less than those of physiologic relevance in whole blood.22
A recent study of fibrin clot structure suggests there is a definitive diagnostic potential of characterizing clot structure and the modulation of clot architecture as a possible treatment for thrombosis.23 As a result, there is a growing need to provide a functional biomarker in terms of a “healthy index” for normal clotting, from which the effect of therapeutic manipulation and disease on clot quality and outcome can be monitored. This was one of the aims of our present study, in which we sought to investigate the value of fractal dimension (Df) characterizing incipient clots formed in samples of whole, unadulterated blood drawn from healthy subjects. The purpose of this investigation was to seek to establish a “healthy” index to represent an optimal value of incipient clot microstructure in terms of Df. Further, by manipulation of the healthy blood by the use of unfractionated heparin, we sought to ascertain whether altering the coagulation pathways and inhibiting thrombin production alters the value of Df. Heparin significantly modifies fibrin assembly and clot structure by its effect on the coagulation pathways, with increasing levels of heparin causing the formation of thicker fibrin fibers,13 and this could be expected to correspond to a lower value of Df because of the concomitantly increased volume of the pore spaces. Another aim of this study involved comparing changes in Df against standard laboratory coagulation markers and thromboelastography (TEG).
Methods
Healthy group
We imposed strict exclusion criteria on the healthy group to ensure all participants could be considered healthy. These criteria eliminated from the study any healthy volunteers who were taking anticoagulant or antiplatelet therapy and who had any personal or family history of a bleeding disorder or thromboembolic diseases as well as any acute illness, cancer, or hepatic and/or renal dysfunction. The healthy group consisted of 52 healthy patients (29 men, 23 women, mean age 33.6 years, range, 20-69 years).
Anticoagulant group
The anticoagulant group consisted of 38 healthy adults sampled from the healthy group (25 men, 13 women, mean age 35.2 years, range, 25-55 years) subject to the same exclusion criterion as the control group. To study the effect of inhibiting thrombin production on the incipient clot, small volumes (< 10 μL) of unfractionated heparin (from stock concentration 1000 IU/mL Monoparin; CP Pharmaceuticals) were added to 20 mL of whole blood collected in vitro, which produced an effective Antifactor Xa (anti-FXa) concentration range 0.05 to 0.80 IU/mL. The comparatively small volume of heparin added to the larger bulk volume of blood (< 0.05% vol) minimized any dilution effect. The heparin and blood were well mixed and transferred immediately to the test instruments. The range of heparin concentration was chosen with reference to the American College of Chest Physicians' Conference on Antithrombotic Therapy Consensus recommendation regarding the monitoring of unfractionated heparin in the treatment of venous thromboembolism.24 The dose of unfractionated heparin was adjusted to prolong the activated partial thromboplastin time (APTT) to a range corresponding to a heparin level of 0.3 to 0.7 U/mL by heparin anti-FXa analysis. In the present study this range corresponded to values of the APTT greater than 60 seconds.
Blood sampling and data collection
This study was undertaken with full ethical approval from the local regulatory ethics committee of South West Wales. The ethical approval was undertaken in 2 stages involving patient information and fully informed written consent in accordance with the Declaration of Helsinki. At all times the study followed the Standards for the Reporting of Diagnostic Accuracy (STARD) guidelines25 on the validation of new diagnostic testing. In all cases blood was taken slowly and atraumatically from the antecubital vein via a 21-gauge butterfly line into a 20-mL syringe.
Laboratory markers
We used 4-mL aliquots of blood from the bulk sample for full blood count (FBC) analysis, which included a platelet count, with samples collected into plastic, full-draw dipotassium ethylenediaminetetraacetic acid Vacuettes (Greiner Bio-One; ref: 454286). FBC was analyzed by the use of a Sysmex XE 2100 (TOA Medical Electronics) automated hematology analyzer within 2 hours of collection. The analyzer was calibrated according to manufacturer's instructions.
A further 4.5 mL of the venous blood sample was used for routine coagulation studies; it was collected into siliconized glass Vacutainers (Becton Dickinson; ref: 367691). Partial thromboplastin (PT), APTT, and total clotting time (TCT) were measured by the use of a Sysmex CA1500 analyzer within 2 hours of collection by scattered light detection (percentage test endpoint method). TCT measurements involved adding 100 μL of thromboclotin to 100 μL of sample. Clauss fibrinogen concentration was determined by addition of 10 μL of sample to 90 μL of Owren buffer followed by addition of 50 μL of thrombin. The time to clot was recorded and the concentration obtained from a standard calibration curve. Fibrinogen calibration was checked against the 2nd International Fibrinogen Standard Version 4 (NIBSC code 96/612). All reagents were obtained from Dade Behring. Analysis of heparin concentration was performed on the Sysmex CA-1500 by the use of a chromogenic anti-Xa assay by Biophen Heparin 3 (Hyphen Biomed) supplied by Quadratech.
Rheometry
The GP26 defines the rheologic transition between an elasticoviscous fluid and a viscoelastic solid. The clot's hemostatic function requires the properties of a viscoelastic solid; thus, the GP identifies the establishment of the incipient clot. At the GP, the elastic and viscous components of the complex shear modulus G* (the dynamic rigidity, G′, and loss modulus, G″, respectively) scale as power-laws in frequency, ω, as G′(ω) ∼ G″ (ω) ∼ ωα. This feature enables the GP to be identified unambiguously by the corresponding frequency independence of the loss tangent, tanδ ( = G″/G′),18-20 where δ represents the phase angle between stress and strain waveforms in small amplitude oscillatory shear measurements and is related to the exponent α as δ = απ/2.
The fractal characteristics of the 3-dimensional network cluster formed at the GP have been extensively studied and are described in various theoretical treatments of polymerization and gelation in a wide range of systems. One such treatment, known as the percolation theory, describes the GP in terms of a connectivity transition. Below the GP, isolated clusters formed from polymerized monomers represent the sol phase, whereas at the GP, a polymeric cluster establishes sufficient connectivity to become “sample-spanning,” thereby conferring elastic solid-like properties upon the system. The percolation theory defines the polymerizing system as macroscopically homogeneous at a length-scale L ≫ ϵ, whereas for L ≪ ϵ the sample-spanning network cluster is a fractal object whose mass M scales with ϵ as M ∼ ϵDf, where Df is the fractal dimension. The value of Df is calculated from analysis of the viscoelastic data at the GP by use of the established relationship27 :
where D is the space dimension (D = 3 herein). The greater the value of Df, the more compact is the network structure, whereas low values of Df correspond to more open/permeable networks.
Aliquots (10 mL) of blood were transferred directly and immediately after sampling (< 60 seconds) to the custom acrylic coaxial cylinder geometry (1-mm shearing gap) of a TA Instruments ARES rheometer.20 All work was conducted at 37°C, and all measurements were made on aliquots of the same sample by the use of similar measuring geometries with identical measuring surfaces and surface preparation procedures. Sequential frequency and FTMS measurements of G′ and G″ were made in the linear viscoelastic regime immediately after sample loading,18-20 and the GP was identified by attainment of frequency independence of tanδ ( = G″/G′). The corresponding value of α was used to calculate Df. The value of Df and the time, TGP, taken to reach the GP were recorded, and the results were correlated with other markers of hemostasis.
TEG
Alongside the rheometric measurements, aliquots from the same bulk blood samples were analyzed by the use of a TEG (Haemoscope 3000 Clot Analyzer).28 All work was conducted at 37°C with the use of plain cuvettes. The TEG data recorded included (1) the “R-time,” that is, the time elapsed between the start of data collection to a pin movement greater than 2 mm on the TEG; (2) the TMA, the time that elapses between the start of data collection to the maximum amplitude of the TEG; (3) the maximum amplitude (MA) value of the TEG; and (4) the clot lysis index LY30: the amplitude at 30 minutes, expressed as a percentage of the MA value.29,30
Statistical analysis
Statistical analysis was performed with the use of Minitab V15 software. Pearson correlation coefficients were calculated on data assumed to be normally distributed. Two-sample differences were calculated by the use of the 2-sample t test. Standard and multiple regression analysis were performed to identify significant relationships between variables, with multicolinearity identified by calculating variance inflation. Throughout, data were assumed to be significant when P was less than .05.31 The rheologic analysis is performed on freshly drawn samples of whole blood. To determine its reproducibility, we calculated the coefficient of variation for both TGP and Df for 3 normal samples and for samples with high and low levels of heparinization. Both parameters were consistent in their measurability with coefficients of variation of less than 5% for all the conditions tested.
Results
Healthy group
Laboratory markers and TEG.
The results of the full blood count (eg, platelets, hematocrit), coagulation screen (fibrinogen [Clauss], PT, APTT, TCT), and TEG (SP, R-time, TMA, and LY30) were within the expected normal ranges for each of the parameters, excluding 4 patients who presented with abnormal values in one or more of the tests, which led to their removal from the study.
GP (TGP) and fractal dimension (Df).
Analysis of the viscoelastic data obtained for samples of whole, unadulterated blood drawn from healthy subjects indicates a clearly defined value of Df, within a narrow range, which represents an index of clotting in health, where Df = 1.74 (± 0.07). We refer herein to this clearly defined value of Df as a healthy index. Data analysis (Table 1) demonstrates that TGP correlated significantly with Df, platelet count and the APTT. The association of Df with the microstructural characteristics of the fibrin network is supported by its significant correlation with fibrinogen. The APTT result is particularly interesting because it is regarded as the most important global measure of the classical intrinsic or common coagulation pathways32 (in this regard, the PT is a relatively insensitive measure). These results suggest that the GP is significantly associated with global coagulation.
Anticoagulant group
Laboratory markers and TEG.
Increased concentrations of unfractionated heparin resulted in a marked increase in all the parameters designed for monitoring clotting time or clot growth with respect to time (PT, APTT, TCT, SP, R-time, and TMA). Concentrations of cells and proteins remained within normal ranges (FBC and fibrinogen [Clauss]).
GP (TGP) and fractal dimension (Df).
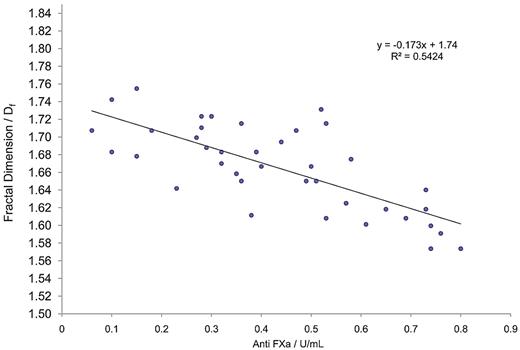
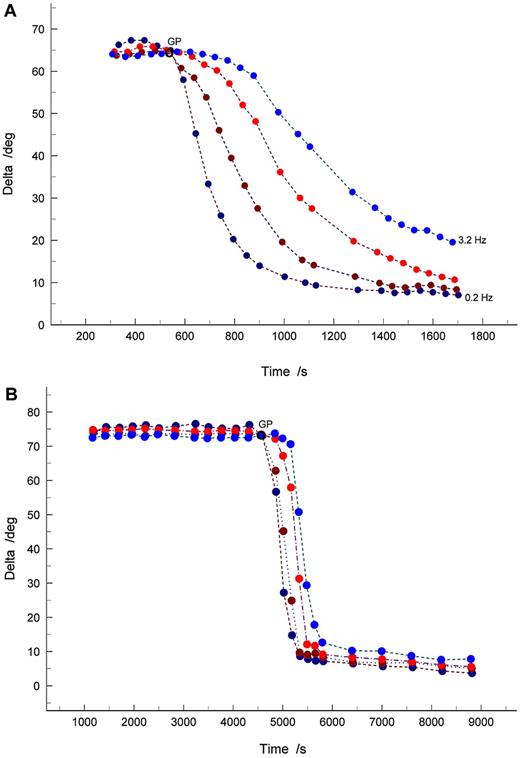
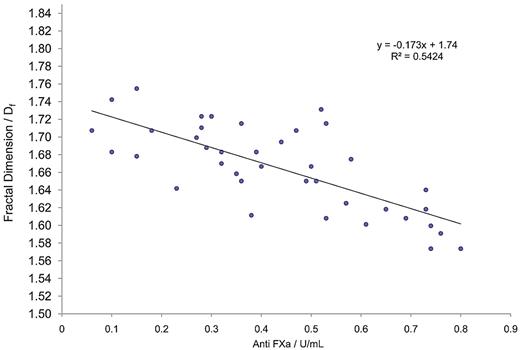
GP analysis further confirmed the existence of significant correlations (P < .05) between TGP, Df, APTT, and also anti-FXa (Table 2; Figure 1). Interestingly, as anti-FXa concentration increased, a decrease in Df was observed, indicating that highly heparinized blood produces incipient clot microstructures that are more open/permeable than those produced at lower heparin concentrations. Figure 2 shows examples of the evolution of δ in samples for which the anti-FXa concentration was 0.52 U/mL (Figure 2A) and 0.76 U/mL (Figure 2B). In viscoelastic systems, δ has values in the range 0° less than δ less than 90°, where 0° and 90° represent the values of δ, which characterize an ideal elastic solid and a Newtonian viscous fluid, respectively. The results illustrate the preincipient clot viscoelastic fluid response, with increasing frequency of oscillation causing δ to decrease. The frequency dependence of δ decreases progressively as coagulation proceeds, becoming frequency independent as the incipient clot is established at the GP. Thereafter, the frequency dependence of δ is characteristic of a viscoelastic solid.
Regression graph of fractal dimension (Df) versus anti-FXa for blood samples treated with heparin. Shown is the best-fit regression curve of Df against FXa calculated for citrated blood samples to which a range of unfractionated heparin had been added (n = 38). A significant relationship is clearly visible, demonstrating the value of measuring fractal dimension during prolonged and abnormal clotting.
Regression graph of fractal dimension (Df) versus anti-FXa for blood samples treated with heparin. Shown is the best-fit regression curve of Df against FXa calculated for citrated blood samples to which a range of unfractionated heparin had been added (n = 38). A significant relationship is clearly visible, demonstrating the value of measuring fractal dimension during prolonged and abnormal clotting.
Examples of the evolution of the phase angle δ over a range of test frequencies identifying the GP where frequency independence of δ is observed. (A) Anti-FXa concentration of 0.52 U/mL, corresponding to Df = 1.69. (B) Anti-FXa concentration of 0.76 U/mL, resulting in Df = 1.58. Oscillatory shear frequencies are 3.2 Hz (light blue circles); 1.0 Hz (red circles); 0.5 Hz (brown circles); and 0.2 Hz (dark blue circles).
Examples of the evolution of the phase angle δ over a range of test frequencies identifying the GP where frequency independence of δ is observed. (A) Anti-FXa concentration of 0.52 U/mL, corresponding to Df = 1.69. (B) Anti-FXa concentration of 0.76 U/mL, resulting in Df = 1.58. Oscillatory shear frequencies are 3.2 Hz (light blue circles); 1.0 Hz (red circles); 0.5 Hz (brown circles); and 0.2 Hz (dark blue circles).
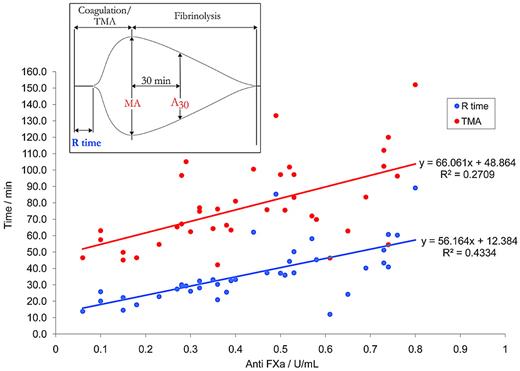
There was a significant prolongation (P < .05; 2-sample t test) in the mean value of TGP in samples treated with heparin (16.28 ± 5.75, mean ± SD) compared with nonheparin-treated samples (4.01 ± 1.63). Furthermore, there was a significant reduction (P < .05; 2-sample t test) in the value of Df for incipient clots formed in the heparinized samples compared with normal samples, with the lowest recorded value of Df (1.58) indicative of a highly tenuous and friable network cluster at the greatest heparin concentration. It is noteworthy that the y-intercept for the best-fit line, (Df = 1.74 for anti-FXa = 0) is highly predictive of the healthy index value found for normal unadulterated blood samples (Df = 1.74 ± 0.07). At concentrations of heparin within the therapeutic range, and up to a concentration of 0.8 U/mL, significant correlation was observed with Df (for which r =−0.733, P < .05; see Figure 2) compared with the TEG R-time (r =−0.527, P < .05; see Figure 3), or the TEG TMA (r = 0.500, P < .05; see Figure 3). It should be noted that the TEG data presented in Figure 3 suggests that the R-time can only be detected accurately by the TEG at low concentrations (< 0.4 U/mL) of anti-FXa, where the reduction in the complexity of clot structure is arguably less affected. The results of a multiregression analysis (which included TEG parameters) revealed Df, together with APTT, to be the most significant predictor of anti-FXa.
Regression graph of TEG R-time and TMA versus anti-FXa for blood samples treated with heparin. The best-fit regression curve of R-time and TMA against anti-FXa calculated for citrated blood samples to which a range of unfractionated heparin had been added (n = 38). For the R-time, a significant relationship was observed (particularly at low concentrations of anti-FXa < 0.4 U/mL), and a general increase in R-time is noted. For the TMA, a significant relationship is demonstrated over the whole range of anti-FXa. Inset: Typical TEG showing the R-time, the TMA, the MA value, and the clot lysis index, or LY30.
Regression graph of TEG R-time and TMA versus anti-FXa for blood samples treated with heparin. The best-fit regression curve of R-time and TMA against anti-FXa calculated for citrated blood samples to which a range of unfractionated heparin had been added (n = 38). For the R-time, a significant relationship was observed (particularly at low concentrations of anti-FXa < 0.4 U/mL), and a general increase in R-time is noted. For the TMA, a significant relationship is demonstrated over the whole range of anti-FXa. Inset: Typical TEG showing the R-time, the TMA, the MA value, and the clot lysis index, or LY30.
Discussion
There have been previous attempts, by authors using various methods, to investigate the relationship between abnormalities or alterations in coagulation pathways and clot quality. A definitive diagnostic potential of characterizing clot structure and the modulation of clot architecture has been suggested,15 and there is an evident need to provide a functional biomarker in terms of a healthy index for normal clotting. The present investigation involving samples of whole, unadulterated blood drawn from healthy subjects, suggests that such a healthy index representing an optimal value of incipient clot microstructure can be established in terms of Df. Moreover, the present study shows how the functional relationship between changes in coagulation pathways and eventual clot outcome in anticoagulated blood can be explored by the use of changes in Df.
Interestingly, the narrow range of values of Df obtained for samples of whole healthy blood (which could be considered the optimum structural template to maximize clot function) is in excellent agreement with predictions of the percolation theory of polymerization and gelation for a heterogeneous system at the sol-gel transition (GP) and similar to that characterizing fractal structures in other biologic systems formed by nonlinear growth processes (eg, the bronchial branching system in the lung).21 In addition, this study demonstrates that by the use of this new rheologic technique, the alteration of fibrin microstructure by manipulation of thrombin production using unfractionated heparin can be recorded over a much wider range, and with greater linearity of response, than by the long-established and widely used technique of TEG.
The finding that a progressive increase in heparin concentration within the therapeutic range results in a corresponding decrease in Df is particularly significant in the context of the incipient clot's role as a microstructural template for ensuing clot development.15 The results reported herein demonstrate that the effect of heparin is not confined to prolongation of the onset of clot formation but that it has a significant effect on clot microstructure. In this respect our results confirm the findings of a previous study of the effect of heparin on fibrin assembly and clot structure.13 That study evaluated the sensitivity to tPA-induced lysis of clots prepared from plasma preincubated in vitro with therapeutic concentrations of heparin. The extent of tPA-induced lysis was significantly increased by preincubation with heparin, and a turbidimetric assay revealed that heparin significantly modified fibrin assembly and clot structure. It was concluded that because of the formation of thicker fibrin fibers, the effect of heparin on clot sensitivity to lysis could be attributed to an increased permeability of the clots to fibrinolytic components. This would correspond to a lower value of Df as a result of the concomitantly increased volume of the pore spaces. The present work is the first to report such an effect of heparin on the fibrin network structure of incipient clots formed in samples of whole blood. In this respect, and given the incipient clot's templating role, it is interesting to note the significant correlation at low concentrations of heparin between Df and the TEG-derived clot lysis index (LY30), which is related to breakdown of the fully formed clot.
The importance of Df in predicting the level of anticoagulation when heparin is added to whole blood in vitro was confirmed by the results of a multiregression analysis (which included TEG parameters), which revealed Df, together with APTT, to be the most significant predictor of anti-FXa. GP and fractal analysis can provide a reproducible measure of clotting status in whole blood in a near-patient setting. The present study suggests that Df and TGP provide significant global markers of hemostasis by determining accurately changes in anticoagulant status of whole blood and determining the functional relationship between pathway changes and eventual clot quality and outcome. The ability of the FTMS technique to provide time-resolved (hence more accurate) measurements of viscoelastic change during coagulation, and an unambiguous determination of the GP, are important rheometric features that could be exploited in future hemorheologic studies to supplant present methods such as the TEG.
The results from this study demonstrate a significant relationship between coagulation pathways and a new, quantitative assessment of the complex, highly disordered microstructure of incipient clots in healthy and anticoagulated blood. Previous attempts by authors to measure clot quality have led to only subjective functional outcomes when clot structure has been associated with the coagulation pathways. Consequently, our findings increase significantly the understanding of clot development in whole blood and introduce a new reproducible measure of clot structure in both normal blood and in blood treated with the anticoagulant heparin. Many disease states affect global hemostasis and clot structure. Our present work is now being extended to clinical studies of thrombotic patients to investigate whether GP analysis and fractal dimension will provide functional biomarkers in such cases.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We grateful to the staff of the Emergency and Hematology departments of the ABMU University Trust Hospital and all the staff of the Clinical Hemostasis and Biomarker Research Unit in Morriston Hospital.
This work was supported by grants from the Engineering and Physical Sciences Research Council (EPSRC) grant (EP/C513037/1; to P.R.W.) and a Royal Society Brian Mercer Feasibility Award (to P.A.E. and P.R.W.).
Authorship
Contribution: P.A.E. and P.R.W. designed the research, analyzed and interpreted the results, and wrote the paper; K.H. and M.J.L. performed all rheometrical and thromboelastogram experiments; R.H.K.M. performed the statistical analysis and interpreted the results; N.T. was responsible for recruiting, consenting, and collecting blood samples from volunteers; and R.M. and L.W. organized all coagulation and full blood count screens.
Conflict-of-interest disclosure: The authors declare no competing financial interests. The EPSRC grant (EP/C513037/1) and the Brian Mercer Feasibility Award were both obtained for translational research for developing techniques from different backgrounds for clinical research.
Correspondence: Prof P. A. Evans, Emergency Department, Morriston Hospital, ABM University NHS Trust, Heol Maes Eglwys, Swansea, SA6 6NL, United Kingdom; e-mail: Phillip.Evans2@wales.nhs.uk; or School of Medicine, Swansea University, Swansea, SA2 8PP, United Kingdom; e-mail: P.A.Evans@swansea.ac.uk.