In this issue of Blood, Walters and colleagues describe an elegant model of WHIM syndrome in the zebrafish embryo. By allowing the movement of WHIM neutrophils to be observed in live animals, this model dramatically illustrates the dynamics of the interaction between the neutrophil chemokine receptor CXCR4 and its receptor ligand (stromal-derived factor-1 [SDF-1], also known as CXCL12) in the hallmark of WHIM-excessive neutrophil adhesion to the marrow stroma.1
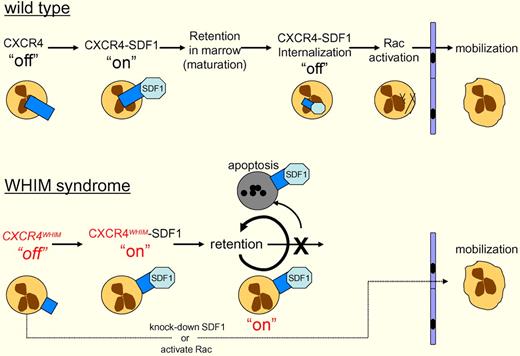
Wild-type neutrophils in the bone marrow express CXCR4 and interact with stromal cells expressing SDF1. This interaction activates CXCR4 (“on”), sending a blocking signal to the cells' motility apparatus, which prevents egress from the marrow. As the neutrophils mature, CXCR4-SDF1 is internalized and degraded (“off”), which leads to Rac activation, allowing the cells to migrate out of the marrow. WHIM syndrome patients express a dominant gain-of-function C-terminal truncation of CXCR4. Walters and colleagues have shown that CXCR4WHIM nonetheless requires the binding of SDF-1 to activate CXCR4WHIM (“on”), blocking motility. CXCR4WHIM is not internalized, so the “on” signal persists, resulting in retention of neutrophils in the marrow, and ultimately apoptosis. This retention can be bypassed, either by knocking down SDF-1, or activating Rac, the G-protein that drives actin polymerization and neutrophil motility.
Wild-type neutrophils in the bone marrow express CXCR4 and interact with stromal cells expressing SDF1. This interaction activates CXCR4 (“on”), sending a blocking signal to the cells' motility apparatus, which prevents egress from the marrow. As the neutrophils mature, CXCR4-SDF1 is internalized and degraded (“off”), which leads to Rac activation, allowing the cells to migrate out of the marrow. WHIM syndrome patients express a dominant gain-of-function C-terminal truncation of CXCR4. Walters and colleagues have shown that CXCR4WHIM nonetheless requires the binding of SDF-1 to activate CXCR4WHIM (“on”), blocking motility. CXCR4WHIM is not internalized, so the “on” signal persists, resulting in retention of neutrophils in the marrow, and ultimately apoptosis. This retention can be bypassed, either by knocking down SDF-1, or activating Rac, the G-protein that drives actin polymerization and neutrophil motility.
SDF-1–mediated activation of CXCR4 underlies routes of cellular travel as diverse as primordial germ cell migration, invasive migration of cancer cells, and leukocyte trafficking from the bone marrow.2-4 Thus, understanding the mechanics of the interaction between these 2 cellular receptors has considerable clinical as well as biologic importance.
The acronym WHIM derives from the constellation of clinical features: Warts (human papilloma virus [HPV]), Hypogammaglobulinemia, Infections (recurrent bacterial), and Myelokathexis (retention of neutrophils in the bone marrow). The immune deficiency stems from several features of the disease: neutropenia from failure of neutrophils to egress from the bone marrow, B-cell lymphopenia, and hypogammaglobulinemia. Treatment generally consists of granulocyte-colony stimulating factor (G-CSF) to release neutrophils from the bone marrow, and injections of gammaglobulin to help resolve the persistent infections. Despite treatment, metastatic carcinomas from HPV, Epstein-Barr virus–based B-cell lymphomas, and bacterial infections remain sources of premature mortality in WHIM patients.5
Most cases of WHIM syndrome arise from dominant mutations in the G-protein–coupled cytokine receptor CXCR4.5,6 These mutations are usually C-terminal truncations creating gain-of-function alleles that lead to increased sensitivity to SDF-1 by preventing the internalization, and subsequent down-regulation, of CXCR4. The mutant CXCR4 results in retention and apoptosis of the neutrophils in the bone marrow, leading to neutropenia (see figure).5,7
Kawai et al first identified the direct role of CXCR4 in myelokathexis in 2007.8 These investigators transduced human CD34+ cells with a CXCR4WHIM allele and transplanted the cells into NOD/SCID mice. They observed that expression of CXCR4WHIM led to neutrophil retention in the bone marrow and increased apoptosis in vivo. While directly linking CXCR4WHIM with the myelokathexis phenotype, the Kawai study did not address the development of mature neutrophils or their homeostasis in the animals that received transplants.
In this study, Walters and colleagues exploit a feature of the zebrafish that has made it the darling of developmental biologists—the external development of the transparent zebrafish embryo—to observe neutrophil dynamics in vivo while manipulating CXCR4 and SDF-1. They first show that zebrafish express CXCR4 and SDF-1 homologues. Next, they derive green fluorescent protein (GFP)–tagged wild-type and C-terminal truncation alleles of zebrafish CXCR4 that mimic human CXCR4WHIM alleles and show that zebrafish CXCR4 localizes to the cell surface and internalizes in response to human SDF-1, whereas CXCR4WHIM fails to internalize when exposed to SDF-1.
Walters et al subsequently generate transgenic zebrafish using the myeloperoxidase promoter driving neutrophil-specific expression of the GFP-CXCR4WHIM allele to show aggregation of neutrophils at the sites of hematopoiesis and other sites of high SDF-1 expression in zebrafish embryos. Knocking down the expression of SDF-1 using morpholino antisense oligonucleotides causes the dispersal of CXCR4WHIM neutrophils. In contrast, ectopic expression of SDF-1 leads to neutrophil aggregation at these sites. Together, these results show that CXCR4WHIM neutrophils accumulate and remain at sites of high SDF-1 expression in vivo.
The transparency of zebrafish embryos allows for the direct visualization of the vasculature in live animals without perturbation. When neutrophil motility is assayed in transgenic embryos expressing GFP-CXCR4WHIM, the GFP-positive cells display greatly reduced 3-dimensional motility compared with GFP-CXCR4 controls. The GFP-CXCR4WHIM animals are also severely neutropenic, like human WHIM patients, with virtually no neutrophils in circulation.
The aggregation of zebrafish CXCR4WHIM neutrophils and their failure to circulate in an SDF-1–dependent manner make it a useful model for WHIM syndrome. The question of clinical importance is how the CXCR4WHIM neutrophils respond to inflammation. By clipping the tailfin, which stimulates neutrophil migration to the wound site, the authors show that the CXCR4WHIM neutrophils fail to migrate to the wound. When SDF-1 expression is inhibited, migration occurs. This is not, however, a trivial result because Walters et al observe that the CXCR4WHIM neutrophils respond to the tailfin clip by increasing cellular protrusion and localized motility. This implies that the neutrophils are not deaf to inflammatory signals, but rather that their motility is in some way restricted by the CXCR4WHIM/SDF-1 interaction. This has not been previously demonstrated in vivo, and it may be an important factor for understanding the excessive neutrophil proliferation and apoptosis in the marrow of WHIM patients.
Walters et al have also begun to dissect the pathway regulating neutrophil motility using a photo-activated Rac GTPase, a downstream component of the SDF-1 signaling pathway. Earlier work suggested WHIM neutrophils are defective in actin polarization.9 Walters and colleagues used this Rac allele to drive actin polymerization, which in turn allowed neutrophil migration to wound sites. These experiments demonstrate a more direct cause-and-effect link between CXCR4 function and the machinery for cellular motility. It is nicely consistent with the observation that CXCR4WHIM neutrophils do, in fact, respond to inflammatory signals, but they cannot mobilize.
The zebrafish WHIM syndrome model presented by Walters et al demonstrates in vivo that truncated alleles of CXCR4, together with its ligand SDF-1, are essential drivers of neutrophil retention in WHIM syndrome. They demonstrate in vivo that the CXCR4 alleles found in WHIM syndrome are a dominant “on” receptor that still requires its ligand, SDF-1, for activation. This “activation” is a STOP signal preventing actin polymerization, and hence retards neutrophil motility out of the marrow into the periphery.
Walters and colleagues are now positioned to use their zebrafish system to investigate how CXCR4WHIM/SDF-1 interactions affect neutrophil homeostasis. The signals driving myelokathexis in WHIM patients remains an important unsolved mystery. This zebrafish WHIM model now provides a tool to screen for agents that either enhance or inhibit neutrophil migration from the bone marrow. The recent identification of zebrafish G-CSF will further enhance the value of this system.10
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
References
National Institutes of Health