Abstract
Lymphotoxin-α (LTα), lymphotoxin-β (LTβ), and tumor necrosis factor-α (TNFα) are inflammatory mediators that play crucial roles in lymphoid organ development. We demonstrate here that LTα also contributes to the function of lymphatic vessels and to lymphangiogenesis during inflammation. LTα−/− mice exhibited reduced lymph flow velocities and increased interstitial fluid pressure. Airways of LTβ−/− mice infected with Mycoplasma pulmonis had significantly more lymphangiogenesis than wild type (WT) or LTα−/− mice, as did the skin draining immunization sites of LTβ−/− mice. Macrophages, B cells, and T cells, known sources of LT and TNFα, were apparent in the skin surrounding the immunization sites as were LTα, LTβ, and TNFα mRNAs. Ectopic expression of LTα led to the development of LYVE-1 and Prox1-positive lymphatic vessels within tertiary lymphoid organs (TLOs). Quantification of pancreatic lymphatic vessel density in RIPLTαLTβ−/− and WT mice revealed that LTα was sufficient for inducing lymphangiogenesis and that LTβ was not required for this process. Kidneys of inducible LTα transgenic mice developed lymphatic vessels before the appearance of obvious TLOs. These data indicate that LTα plays a significant role in lymphatic vessel function and in inflammation-associated lymphangiogenesis.
Introduction
Recent years have seen great advances in the molecular understanding of lymphatic vessels and lymphangiogenesis.1 Studies with genetically engineered mice identified several key growth factors, transcription factors, transmembrane glycoproteins, and signaling proteins that are crucial for lymphatic vessel development and function.2-5 Varying deficiencies in these critical molecules resulted in a spectrum of lymphedema phenotypes ranging from edematous embryos with chylous ascites and severe vascular defects associated with perinatal lethality,3,6 to more subtle manifestations of lymphatic function defects.7-9 Derangements in the structure or function of lymphatic vessels may induce edema.1 Chy mice, in which a heterozygous mutation in the Vegfr3 gene results in inactivation of vascular endothelial growth factor 3 (VEGFR-3) signaling, exhibit lymphedema only in the fore and hind paws despite an absence of lymphatics in the entire dermis, thus demonstrating the effectiveness of compensatory mechanisms.9
Lymphangiogenesis has been shown to occur at the sites of inflammation in models of corneal transplantation and airway infection.10-12 During chronic inflammation in autoimmunity, graft rejection and infection, chronic accumulations of lymphoid cells that resemble lymph nodes, termed “tertiary lymphoid organs” (TLOs) develop, many of which require members of the lymphotoxin (LT)/TNF family.13 Lymphangiogenesis has been described in TLOs in thyroiditis, sialitis, rheumatoid arthritis, and chronic kidney graft rejection.14-17
LTs, key mediators of inflammation through the induction of chemokines and vascular adhesion molecules,18 also play crucial roles in lymphoid organ development.19 The homotrimer LTα3 is secreted by CD4+ Th1, CD8+, NK, B, and lymphoid tissue inducer cells and signals through TNFR1 and TNFR2, explaining its partial redundancy to TNFα3, which signals through the same receptors.20 TNFα is made by a wider variety of cells, including macrophages, in addition to the LTα-producing lymphocytes. The LTα monomer also forms a heterotrimer with LTβ that is required for the cell surface expression of the LTαβ complex.21 The LTα1β2 complex binds to and signals through the LTβR. Mice deficient in LTα lack all lymph nodes and Peyer patches,19 whereas those deficient in LTβ retain some cervical, sacral, and mesenteric lymph nodes.22
Transgenic expression of mouse LTα under the control of the rat insulin promoter II (RIP) leads to its expression in the β cells of the islets of Langerhans in the pancreas as expected, and in the skin23 and proximal convoluted tubules of the kidney23,24 as this promoter is somewhat “leaky.” These mice develop accumulations of T and B cells, dendritic cells, follicular dendritic cells, and macrophages that are organized into TLOs that resemble lymph nodes in cellular composition and compartmentalization, the presence of high endothelial venules (HEVs) and lymphoid chemokine expression,18,25 due to signaling through TNFR1.26 RIPLTβ mice have no apparent phenotype, but when crossed to RIPLTα mice (RIPLTαβ mice), develop more extensive cellular infiltrates in the pancreas and kidney, and a more mature HEV phenotype due to signaling through the LTβR.27 LTβ has been implicated in lymphangiogenesis in a transgenic thyroid TLO model.15 In addition, treatment of mouse embryo fibroblasts in vitro with an agonistic LTβR mAb induces VEGF-C mRNA,28 suggesting that LTα1β2 is capable of inducing a lymphangiogenic factor.
Despite its known roles in lymph node development, inflammation and TLO formation, the contribution of LTα, distinct from the LTαβ complex, to lymphatic vessel development and function has yet to be evaluated. We were interested in whether LTα could affect lymphangiogenesis and postulated that LTs may induce cellular infiltrates that could influence this process. Furthermore, because TNFα induces changes in lymphatic endothelial cells in vitro29 and has been implicated in lymphangiogenesis in inflamed airways,11 it is likely that LTα behaves similarly as it exerts effects analogous to those of TNFα on blood endothelial cells in vitro.30 Here we evaluate the effect of LTs on lymphatic vessels in vivo. We show for the first time that mice deficient in LTα, but not LTβ, have functional deficiencies in their lymphatic vasculature. We also demonstrate in several models of inflammation that LTα promotes lymphangiogenesis and that the LTα1β2 complex is not essential for this process.
Methods
Mice
C57BL/6 wild type (WT) and C57BL/6-Tnfrsf1atm1Imx/J (TNFR1−/−) mice were purchased from The Jackson Laboratory. RIPLTα, LTα−/−, and LTβ−/− mice were maintained at Yale University19,22,25 under specific pathogen-free conditions or shipped to University of California, San Francisco (UCSF) or the University of Bergen. RIPLTαLTβ−/− mice were generated by crossing RIPLTα mice to LTβ−/− mice and then backcrossing to LTβ−/− mice. These mice were screened by polymerase chain reaction (PCR) to ensure the absence of LTβ. In most experiments, male and female mice between 8 and 12 weeks of age were used. Due to issues involving international transport and acclimation, transcapillary fluid balance and lymphatic vessel function were measured in mice at 7 to 11 months of age. Most studies were done under a protocol approved by the Yale University Institutional Animal Care and Use Committee. Residence time distribution studies and Mycoplasma experiments were approved by the Institution Animal Care and Use Committees of Massachusetts General Hospital (MGH) and UCSF, respectively. Transcapillary fluid balance and lymphatic function studies were approved by, and carried out, in accordance with the Norwegian State Commission for Laboratory Animals.
Production of RIPLTαTetOn mice
Mice conditionally inducible for LTα expression were transgenic for 2 constructs. The RIPrtTA construct contained the rat insulin promoter driving rtTA and human growth hormone intronic and polyadenylation signals. rtTA is a fusion protein made up of a tetracycline repressor and the herpes virus transactivator. The TetopLTα construct consisted of a polymeric tetracycline operator (Tetop), the mouse LTα gene, and the human growth hormone (hGH) intronic and polyadenylation sequences. In this system, the RIP promoter should direct the expression of rtTA in the pancreas, kidney, and skin.23 In the presence of doxycycline (dox), rtTA binds in trans to the Tet-operon, and VP-16 transactivates LTα transcription.23,25 This construct was ligated to a construct consisting of a fragment of plasmid 172.1neo, a gift of H. Bujard (University of Heidelberg), which was then modified with an hGH gene.31,32 The TetopLTα construct was prepared with the LTα fragment ligated to a construct that consisted of the Tet-operon with a minimal cytomegalovirus (CMV) promoter and hGH intronic and polyadenylation sequences.23,25,31 Transgenic mice were prepared in (CBAxC57BL/6) F2 eggs by simultaneously injecting the constructs into pronuclei. Southern blot analysis revealed progeny positive for both transgenes.
Doxycycline administration
RIPLTαTetOn transgenic mice were maintained on normal food and water until transgene activation was desired. Mice were then fed grain-based doxycycline pellets (Dox Diet, 200 mg/kg; Bio-Serv).
Residence time distribution theory
To measure network lymph flow velocity, mice were anesthetized and their tails were treated with Nair hair remover (Church and Dwight Co) for 10 minutes and then rinsed with water. On the following day, the mice were immobilized on a fiberglass platform using double-sided tape and fluorescein isothiocyanate-dextran (mol wt 2 × 106 Da; Sigma-Aldrich) at a concentration of .025 mg/mL in .9% normal saline (Abbott Laboratories) was injected intracutaneously as a continuous infusion into the tail using a 30G needle at a fixed pressure of 40 cm of H20. The tail was imaged every 10 minutes for 2 hours after the beginning of the injection to monitor the proximal transport of the dextran by the tail lymphatics. The images were then analyzed to calculate network lymph velocity.33
Interstitial fluid pressure and extracellular fluid volume measurement
Mice were anesthetized and body temperature was kept constant at 37°C with a heating pad and lamp. The mice were then catheterized in 1 or both jugular veins for intravenous infusions. Interstitial fluid pressure (Pif) was measured in the hind paw skin by a micropuncture technique using sharpened glass capillaries connected to a servo-controlled counter-pressure device.34 Punctures were performed through intact skin on the dorsum of the hind limb under the visual guidance of a stereomicroscope. The extracellular fluid volume (EFV) was measured using 51Cr-EDTA (3.7 MBq/mL; GE Healthcare), which distributes into the entire extracellular fluid phase. Tracer was injected intravenously as a .13 mL bolus, and thereafter, as a continuous intravenous infusion at a rate of 1.5 μL/minute. A blood sample was obtained by cardiac puncture 90 minutes later. After coagulation, the samples were spun at 10 000g for 10 minutes and serum was collected. Tissue samples from hind paw skin were put in preweighed vials. After reweighing the vials, radioactivity was measured using a gamma counter (COBRA II, AUTO-GAMMA; Packard, Perkin Elmer) with window settings of 240 to 400 keV and appropriate background correction. Samples were then dried at 50°C to achieve a constant weight (ie, dry weight). EFV was calculated as the plasma equivalent distribution volume of 51Cr-EDTA ((cpm/g dry weight)/(cpm/mL plasma)).
Isolation of interstitial fluid and colloid osmotic pressure determination
Interstitial fluid was isolated from skin using a centrifugation technique.35 Thigh skin was then placed on a nylon mesh (15-20 μm pore size) over an Eppendorf tube. After centrifugation at 424g for 10 minutes, fluid representative of interstitial fluid accumulated at the bottom of the tube. Colloid osmotic pressure (COP) was measured in thigh skin interstitial fluid and plasma using a colloid osmometer designed for submicroliter samples.
Challenge of the lymphatic system by overhydration
Overhydration was induced by an intravenous infusion of Ringer-acetate solution. A volume corresponding to 15% of the mouse weight was administered over a period of 1 hour using an infusion pump. Skin samples from hind paws were then excised for EFV measurements. Skin from the opposite thigh was taken for interstitial fluid isolation by centrifugation and COP determination. For the overhydrated mice, Pif was measured at both steady state and after fluid infusion.
M pulmonis infection of airways
WT, LTα −/−, and LTβ−/− mice were infected by intranasal inoculation of 50 μL of broth containing 3.3 × 105 CFU of M pulmonis organisms of strain CT7 and studied 14 days later.11 Corresponding pathogen-free mice were used as controls.
Skin immunization and lymphatic vessel imaging
Mice were anesthetized with 250 μL intraperitoneal injections of ketamine (10 mg/mL) and xylazine (1 mg/mL; Henry Schein) in phosphate-buffered saline (PBS) and both flanks were shaved with electric clippers. Mice were then injected subcutaneously with a total of 375 μg ovalbumin (Grade 5; Sigma-Aldrich) in PBS, mixed with 450 μg of Mycobacterium tuberculosis (Difco, BD Biosciences) in 100 μL complete Freund adjuvant (CFA) into both flanks and tail.36 Lymphatic vessels around immunization sites were imaged using a fluorescein conjugated nanoparticulate contrast agent as previously described.37
Immunohistochemistry
Mice were perfused with 1% paraformaldehyde for 2 minutes and tracheas were harvested and placed in fixative for 1 hour. Whole mount immunostaining for LYVE-1, rabbit polyclonal; 1:1000 (Upstate Millipore), CD31, hamster clone 2H8; 1:500 (Chemicon Millipore) and CD11b, rat clone M1/70 (eBioscience) was performed on whole mount preparations of tracheas.11 Species-specific secondary antibodies labeled with Cy3, Cy5, or FITC were then used; 1:500 (Jackson ImmunoResearch Laboratories).11
Pancreas and kidney tissues were embedded in Tissue-Tek OCT (Sakura Finetek USA), then 7 μm sections were cut and fixed in cold acetone. Background staining was blocked with 5% BSA (Sigma-Aldrich) and 4% goat serum (Sigma-Aldrich) in PBS. Immunostaining for LYVE-1, rabbit IgG; 1:1000 (Upstate) or monoclonal rat IgG; 1:1000 (R&D Systems), Prox1, rabbit IgG; 1:100 (AbCam), B220, CD4 and CD8 rat IgG; 1:250 (BD Biosciences) was then performed. DyLight 549 donkey anti–rabbit IgG; 1:1000 (Jackson ImmunoResearch) was used for the Prox1 staining in the pancreas. Species-specific Cy2 and Cy3-conjugated secondary or tertiary antibodies; 1:1000 (Jackson ImmunoResearch) were then applied. Sections were counterstained with Hematoxylin (Vector Laboratories) or DAPI (Sigma-Aldrich) and mounted with Fluorosave (Calbiochem, EMD Chemicals).
Flow cytometric analysis
After euthanasia, flanks of immunized mice were shaved and skin was harvested around antigen injection sites. Antigen depots were pierced with a needle and emptied. Tissues were then finely minced and incubated at 37°C for 2 hours in digestion buffer (Hepes-buffered RPMI 1640 containing collagenase IV (Worthington Biochemical), DNAse and hyaluronidase (Sigma-Aldrich), and sodium pyruvate (Invitrogen).38 Cells were separated from tissue debris by straining the suspension through a 40 μm nylon mesh. Cells were washed twice with Hanks Balanced Salt Solution (Invitrogen), counted and stained with fluorescent antibodies. Flow cytometry was performed using a FACScalibur flow cytometer (BD Biosciences).
RT-PCR
Total RNA was extracted from the cells of the skin, lymph nodes and spleen using the RNeasy Mini Kit (QIAGEN). First-strand cDNA was synthesized from DNase-treated RNA from each sample using oligo (dT)12-18 and Superscript II reverse transcriptase (RT; Invitrogen). cDNA was used for PCR amplifications using gene-specific primer sets and conditions.39 Reactions were performed using a PTC-100 thermal cycler (MJ Research).
Lymphatic vessel quantification
Sections of 7 μm thick pancreatic tissue were stained for LYVE-1 as described in the staining protocol above. At least 75 sections were cut from each group of WT, RIPLTα, and RIPLTαLTβ−/− mice and 40× images of at least 40 islets were photographed with a microscope (Axioskop; Carl Zeiss). Area densities of LYVE-1 positive vessels were calculated using MetaMorph Version 6.2r6. In the tracheas, area densities of LYVE-1 and CD31 positive vessels were calculated as previously described.11
In situ hybridization
Kidneys from RIPLTαTetOn mice were incubated in 4% paraformaldehyde/.14M Sorenson solution overnight at 4°C. Tissues were embedded in Tissue-Tek OCT (Sakura Finetek USA) and sections were placed on poly-L-lysine coated slides. In situ hybridization was performed using digoxigenin (DIG)–labeled sense and antisense riboprobes for LTα.27 An alkaline-posphatase-conjugated sheep anti-DIG antibody (Roche Diagnostic System) was used for detection and NBT/5-bromo-4-chloro-3-indolyl phosphate (Invitrogen) for development.
Statistics
Values are presented as means plus or minus SEM with 4 to 5 mice per group unless otherwise indicated. For extracellular fluid volume (EFV), Pif, and COP measurements, Mann-Whitney tests for 2 independent samples were used. For the studies on M pulmonis–infected mice, the significance of differences between means was assessed by analysis of variance (ANOVA) followed by the Dunn-Bonferroni test for multiple comparisons. For studies on residence time distribution theory, the data were analyzed with ANOVA by the Tukey Honestly-Significant-Difference Test (post hoc) using Systat 12 from Systat Software Inc. For all analyses, P values less than .05 were considered significant.
Results
LTα−/− lymphatic vessels exhibit decreased lymph flow velocity
Defects in the lymphatic vasculature typically manifest as lymphedema, which may be difficult to assess grossly in mice.40 Because we did not observe any overt edema in LT deficient mice upon examination of the paws and limbs, we turned to sensitive and quantifiable techniques for evaluating lymphatic vessel function.33-35 Residence time distribution theory is a standard method to determine network lymph flow velocities in the tail.33,41 This robust, quantitative technique allowed us to evaluate lymph flow in the distal tail where no lymph nodes are present and, therefore, could not influence lymph flow velocity. In comparison to WT mice, LTα−/− mice exhibited a significant decrease in lymph flow velocity of approximately 40% (Figure 1A). This effect was not apparent in the LTβ−/− mice in our experiments powered to detect a biologic difference of 25%. Thus, LTα appears to play a critical role in the normal function of lymphatic vessels.
Analysis of lymph flow velocity and physiology. (A) Quantitative analysis using residence time distribution theory reveals a significant difference in the lymph flow velocity of fluorescent dextran in tail lymphatics between LTα−/− and wild type (WT) mice, but not between LTβ−/− and WT mice (a minimum of n = 10 mice per group; *P < .05). (B) Extracellular fluid volume (EFV) in paw skin measured during steady state control situation ( ) and after overhydration (
) and after overhydration ( ). In contrast to LTα−/− mice (n = 8 in control [c] and overhydration [o]), both LTβ−/− (n = 8 [c] and n = 9 [o]) and WT (n = 6 [c] and n = 4 [o]) mice show a significant increase in EFV after overhydration (*P < .05). (C) LTα−/− mice (n = 16) have a significantly higher interstitial fluid pressure (Pif) than both LTβ−/− (n = 17) and WT mice (n = 10) during steady state control situation (*P < .05). All 3 groups, however, demonstrate a significant increase in Pif after overhydration (#P < .05). (D) Colloid osmotic pressure (COP) in plasma and interstitial fluid in the steady state control situation. LTα−/− mice show comparable COP in the interstitial fluid to WT and LTβ−/− mice. (E) After overhydration, the LTα−/− mice (n = 6) show a significantly higher COP in the interstitial fluid in comparison to WT mice (n = 7; *P < .05). (F) Colloid osmotic pressure gradient (ΔCOP) across the capillaries in the steady state control situation and after overhydration. All 3 groups show a nonsignificant decrease in ΔCOP after overhydration. Overhydrated LTα−/− (n = 6) mice have a significantly lower ΔCOP compared with WT (n = 7) mice (*P < .05).
). In contrast to LTα−/− mice (n = 8 in control [c] and overhydration [o]), both LTβ−/− (n = 8 [c] and n = 9 [o]) and WT (n = 6 [c] and n = 4 [o]) mice show a significant increase in EFV after overhydration (*P < .05). (C) LTα−/− mice (n = 16) have a significantly higher interstitial fluid pressure (Pif) than both LTβ−/− (n = 17) and WT mice (n = 10) during steady state control situation (*P < .05). All 3 groups, however, demonstrate a significant increase in Pif after overhydration (#P < .05). (D) Colloid osmotic pressure (COP) in plasma and interstitial fluid in the steady state control situation. LTα−/− mice show comparable COP in the interstitial fluid to WT and LTβ−/− mice. (E) After overhydration, the LTα−/− mice (n = 6) show a significantly higher COP in the interstitial fluid in comparison to WT mice (n = 7; *P < .05). (F) Colloid osmotic pressure gradient (ΔCOP) across the capillaries in the steady state control situation and after overhydration. All 3 groups show a nonsignificant decrease in ΔCOP after overhydration. Overhydrated LTα−/− (n = 6) mice have a significantly lower ΔCOP compared with WT (n = 7) mice (*P < .05).
Analysis of lymph flow velocity and physiology. (A) Quantitative analysis using residence time distribution theory reveals a significant difference in the lymph flow velocity of fluorescent dextran in tail lymphatics between LTα−/− and wild type (WT) mice, but not between LTβ−/− and WT mice (a minimum of n = 10 mice per group; *P < .05). (B) Extracellular fluid volume (EFV) in paw skin measured during steady state control situation ( ) and after overhydration (
) and after overhydration ( ). In contrast to LTα−/− mice (n = 8 in control [c] and overhydration [o]), both LTβ−/− (n = 8 [c] and n = 9 [o]) and WT (n = 6 [c] and n = 4 [o]) mice show a significant increase in EFV after overhydration (*P < .05). (C) LTα−/− mice (n = 16) have a significantly higher interstitial fluid pressure (Pif) than both LTβ−/− (n = 17) and WT mice (n = 10) during steady state control situation (*P < .05). All 3 groups, however, demonstrate a significant increase in Pif after overhydration (#P < .05). (D) Colloid osmotic pressure (COP) in plasma and interstitial fluid in the steady state control situation. LTα−/− mice show comparable COP in the interstitial fluid to WT and LTβ−/− mice. (E) After overhydration, the LTα−/− mice (n = 6) show a significantly higher COP in the interstitial fluid in comparison to WT mice (n = 7; *P < .05). (F) Colloid osmotic pressure gradient (ΔCOP) across the capillaries in the steady state control situation and after overhydration. All 3 groups show a nonsignificant decrease in ΔCOP after overhydration. Overhydrated LTα−/− (n = 6) mice have a significantly lower ΔCOP compared with WT (n = 7) mice (*P < .05).
). In contrast to LTα−/− mice (n = 8 in control [c] and overhydration [o]), both LTβ−/− (n = 8 [c] and n = 9 [o]) and WT (n = 6 [c] and n = 4 [o]) mice show a significant increase in EFV after overhydration (*P < .05). (C) LTα−/− mice (n = 16) have a significantly higher interstitial fluid pressure (Pif) than both LTβ−/− (n = 17) and WT mice (n = 10) during steady state control situation (*P < .05). All 3 groups, however, demonstrate a significant increase in Pif after overhydration (#P < .05). (D) Colloid osmotic pressure (COP) in plasma and interstitial fluid in the steady state control situation. LTα−/− mice show comparable COP in the interstitial fluid to WT and LTβ−/− mice. (E) After overhydration, the LTα−/− mice (n = 6) show a significantly higher COP in the interstitial fluid in comparison to WT mice (n = 7; *P < .05). (F) Colloid osmotic pressure gradient (ΔCOP) across the capillaries in the steady state control situation and after overhydration. All 3 groups show a nonsignificant decrease in ΔCOP after overhydration. Overhydrated LTα−/− (n = 6) mice have a significantly lower ΔCOP compared with WT (n = 7) mice (*P < .05).
LTα affects transcapillary fluid balance and lymphatic vessel function
Exploring the changes in lymphatic flow velocity further, we examined the physiologic ramifications of reduced lymphatic vessel function caused by a deficiency in LTα. Tissue compliance, COP in plasma (p) and interstitial fluid (if) and lymph flow, all represent additional determinants of tissue fluid balance and ultimately lymphatic vessel function.42 We measured the EFV in the skin of male mice and found the EFV to be similar in LTα−/−, LTβ−/− and WT mice in the steady state control condition (Figure 1B white bars). During steady state control conditions however, the Pif in LTα−/− mice was significantly higher compared with LTβ−/− and WT mice (Figure 1C). The higher Pif represents an increased filling pressure for the lymphatic vessels, which most likely is a consequence of, and thus a compensatory mechanism for, the reduced lymph removal rate in LTα−/− mice (Figure 1A). In contrast, there was no significant difference in either COPp or COPif among these mice (Figure 1D). Upon exposure to a fluid challenge, induced by the infusion of Ringer-acetate solution, the Pif increased in all mouse strains and the difference in pressure in the different strains resolved (Figure 1C). Interestingly, although the COPif in the thigh skin fell in all strains after infusion, the pressure stabilized at higher levels in LTα−/− mice compared with WT mice (Figure 1E). Moreover, the net COP gradient, that is, COPp minus COPif, across the capillaries was significantly lower in the LTα−/− mice after overhydration in comparison to WT mice (Figure 1F). These differences were also reflected in the measured ECV in the paw skin, where LTβ−/− and WT mice had a significant increase in ECV after overhydration; such an increase was not observed in LTα−/− mice (Figure 1B black bars). The higher absolute COPif, the lower net COP gradient and unchanged EFV upon overhydration in LTα−/− mice can all be explained by the increased Pif counteracting fluid movement across the capillaries. Using regression analysis,42 we found a significant linear relation between ECV and Pif in LTβ−/− and WT mice. The changes in Pif resulting from the induced changes in volume were comparable, suggesting that tissue compliance was similar in these strains. A linear volume-pressure relationship was, however, not found in LTα−/− mice (data not shown). Taken together, these experiments demonstrate that mice deficient in LTα activate autoregulatory mechanisms, notably an increased Pif, to compensate for the defective lymphatic function in this strain.42
Differential roles for LTα and LTβ in the induction of lymphangiogenesis in inflammation
The data presented in the sections above suggested that the absence of LTα resulted in a deficiency in lymphatic vessel function. To further probe the roles of the LT family in lymphangiogenesis, we evaluated 2 different models of inflammation. Lymphangiogenesis during states of inflammation has been clearly described. Skin painting with the inflammatory irritant oxazolone induces an increase in lymphatic vessel density in the skin of WT mice.36 Infection with M pulmonis also induces both lymphangiogenesis and angiogenesis in the airways of infected mice.11 In addition, CD11b positive macrophages were shown to play a role in lymphangiogenesis during inflammatory states in both the trachea and cornea, with important roles for VEGF-C and D.10,11
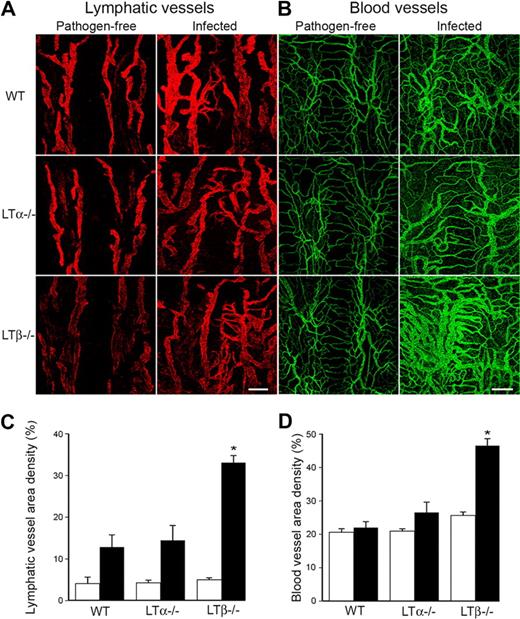
We infected WT, LTα−/− and LTβ−/− mice with M pulmonis and assessed lymphangiogenesis and angiogenesis in their tracheas. WT mice demonstrated growth of lymphatic and blood vessels in the trachea after infection showing enlarged vessels and lymphatic sprouts as expected from our previous publications11,43,44 (Figures 2A-B). LTα−/− mice demonstrated a similar response, whereas the LTβ−/− mice, surprisingly, developed more extensive lymphatic and blood vessel networks (Figures 2A-B). These remodeled blood and lymphatic vessels developed in regions heavily infiltrated with CD11b-positive cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Quantification revealed a significantly greater increase in both lymphatic (Figure 2C) and blood vessel (Figure 2D) densities in LTβ−/− mice in comparison to LTα−/− and WT mice.
Increased lymphangiogenesis in LTβ−/− mouse tracheas after infection with Mycoplasma pulmonis. (A) LYVE-1+ lymphatic vessels (red) and (B) CD31+ blood vessels (green) increase in the tracheas of mice infected with M pulmonis. Scale bar is 200 μm (Zeiss 510 Confocal Microscope). (C) Quantification reveals a significant increase in lymphatic vessel density in the tracheas of LTβ−/− mice compared with both LTα−/− and WT mice (*P < .05). (D) A similar increase was noted in blood vessel density.
Increased lymphangiogenesis in LTβ−/− mouse tracheas after infection with Mycoplasma pulmonis. (A) LYVE-1+ lymphatic vessels (red) and (B) CD31+ blood vessels (green) increase in the tracheas of mice infected with M pulmonis. Scale bar is 200 μm (Zeiss 510 Confocal Microscope). (C) Quantification reveals a significant increase in lymphatic vessel density in the tracheas of LTβ−/− mice compared with both LTα−/− and WT mice (*P < .05). (D) A similar increase was noted in blood vessel density.
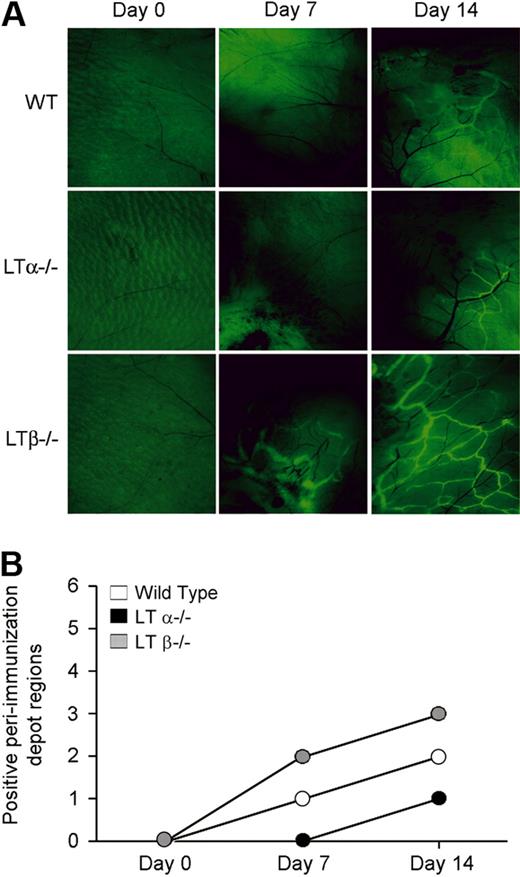
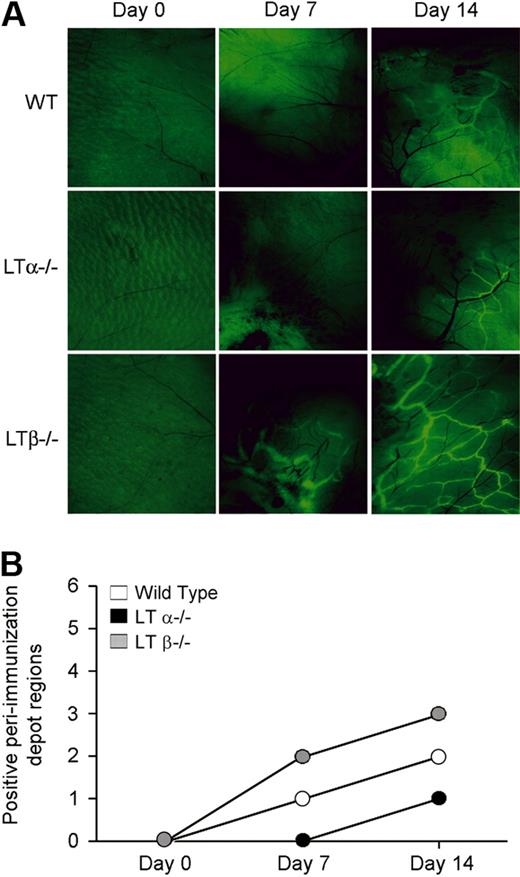
We next evaluated the role of LTα in lymphangiogenesis using a soluble protein antigen immunization model of inflammation. We immunized WT, LTα−/− and LTβ−/− mice subcutaneously with ovalbumin and CFA in both flanks to induce an inflammatory response. Lymphangiogenesis was evaluated using fluorescent nanoparticles that were injected subcutaneously into the footpad and taken up by the lymphatic vasculature.37 LTβ−/− mice demonstrated more uptake of nanoparticles and had more extensive lymphatic vessel networks in the subcutaneous tissue near the immunization sites than did the LTα−/− and WT mice. These lymphatics were more widespread and were evident earlier than in WT or LTα−/− mice, where the lymphatics were only seen at a later time, that is, at day 14 (Figure 3A). In addition, lymphatic vessels were found in more peri-immunization regions in immunized LTβ−/− mice than either LTα−/− or WT mice (Figure 3B). Lymphatics were not seen around immunization sites in TNFR1−/− mice (supplemental Figure 2). Taken together, these data suggest that LTβ expression results in reduced lymphangiogenesis and that LTα, in the form of LTα3, contributes to lymphatic vessel development through TNF receptors.
Increased lymphangiogenesis in LTβ−/− mouse skin after induction of inflammation. (A) Fluorescence microscopy of the site of immunization with ovalbumin and CFA after injection of FITC-conjugated nanoparticles reveals more prominent and extensive lymphatic vessel networks near immunization sites in LTβ−/− mice compared with LTα−/− and WT mice. Pale green at day 0 represents autofluorescence. Black blood vessels are apparent and even more obvious after immunization. Lymphatic vessels are bright green (6.5× objective). (B) More peri-immunization depot sites showed uptake of the nanoparticles by lymphatics in LTβ−/− than WT and LTα−/− mice (n = 3 per group with total of 6 depot regions per group).
Increased lymphangiogenesis in LTβ−/− mouse skin after induction of inflammation. (A) Fluorescence microscopy of the site of immunization with ovalbumin and CFA after injection of FITC-conjugated nanoparticles reveals more prominent and extensive lymphatic vessel networks near immunization sites in LTβ−/− mice compared with LTα−/− and WT mice. Pale green at day 0 represents autofluorescence. Black blood vessels are apparent and even more obvious after immunization. Lymphatic vessels are bright green (6.5× objective). (B) More peri-immunization depot sites showed uptake of the nanoparticles by lymphatics in LTβ−/− than WT and LTα−/− mice (n = 3 per group with total of 6 depot regions per group).
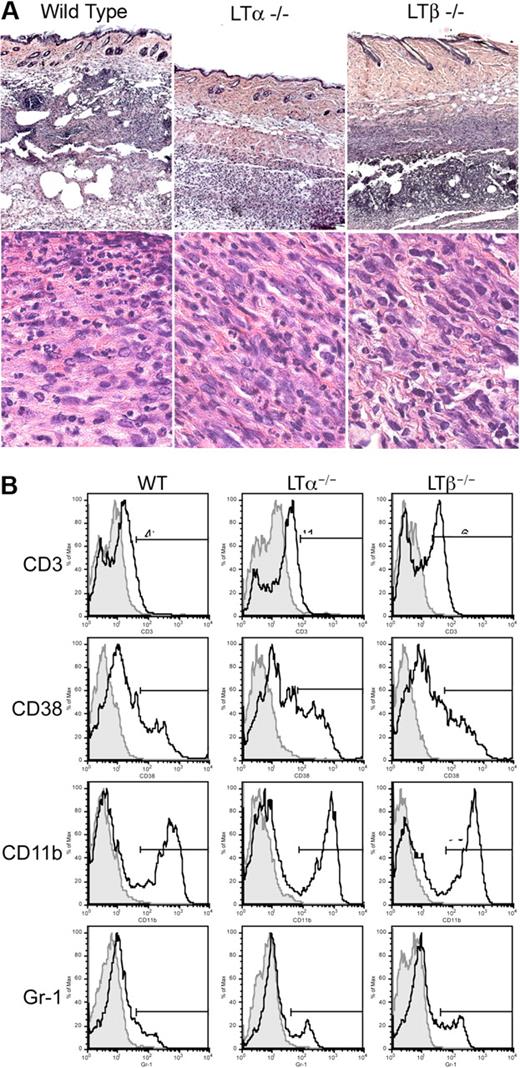
In order to further evaluate the effect of LTα on lymphangiogenesis, we investigated whether cells in the skin surrounding the immunization site produced LTα, LTβ, and TNFα. Histologic analysis of immunized skin regions revealed extensive cellular infiltrates, which included mononuclear cells with the appearance of macrophages and neutrophils (Figure 4A). FACS analysis of living cells isolated from WT, LTα−/− and LTβ−/− skin at days 4 and 7 after immunization revealed heterogeneous populations of cells that stained for Gr-1 (granulocytes), CD11b (macrophages and neutrophils), CD38 (B cells), and CD3 (T cells; Figure 4B). The infiltrates, therefore, included several populations of cells capable of producing LTα and LTβ (T cells and B cells) and TNFα (T cells, macrophages, and B cells). There were also consistently higher percentages of macrophages and granulocytes in LTβ−/− skin in comparison to WT and LTα−/− mice, consistent with the increased lymphangiogenesis in LTβ−/− mice.
Massive leukocytic infiltrates at immunization sites in the skin. (A) Hematoxylin and eosin staining of the skin at the site of immunization with ovalbumin and CFA on day 7 reveals cellular infiltration of the skin. (original magnifications 5× or 100× oil objective; Zeiss Axioscope). (B) FACS analysis of cells isolated from skin site of immunization reveals consistently increased proportions of macrophages and granulocytes in skin from LTβ−/− mice (representative of 4 experiments at days 4 and 7; n = 4 mice per group).
Massive leukocytic infiltrates at immunization sites in the skin. (A) Hematoxylin and eosin staining of the skin at the site of immunization with ovalbumin and CFA on day 7 reveals cellular infiltration of the skin. (original magnifications 5× or 100× oil objective; Zeiss Axioscope). (B) FACS analysis of cells isolated from skin site of immunization reveals consistently increased proportions of macrophages and granulocytes in skin from LTβ−/− mice (representative of 4 experiments at days 4 and 7; n = 4 mice per group).
RT-PCR performed on the skin of WT mice at 7 days after immunization revealed that the infiltrating cells produced cytokines of all 3 members of the LT/TNF family. As expected, mRNA for LTα was not apparent in the LTα−/− mouse skin and LTβ mRNA was not apparent in LTβ−/− mouse skin. However, very high levels of LTα mRNA were detectable in the skin of the LTβ−/− mice (supplemental Figure 3). These findings are consistent with the interpretation that, in the absence of LTβ, more LTα is available in both the M pulmonis model and the skin immunization model to form LTα3 homotrimers that bind to TNF receptors and complement the activity of TNFα.14
Transgenic expression of LTα results in lymphangiogenesis in tertiary lymphoid organs
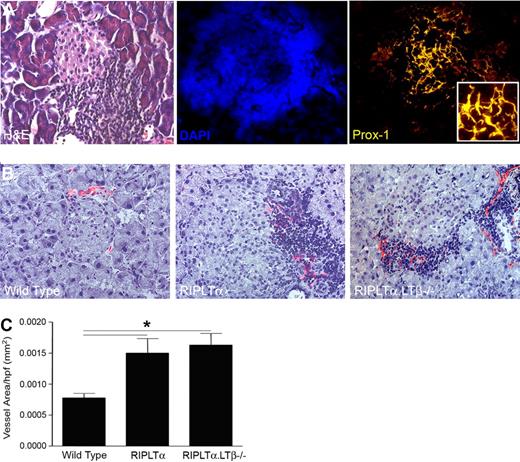
Cellular accumulations resembling TLOs developing in chronically rejecting human kidneys are associated with a significant increase in lymphatic vessel density.17 Therefore, we next asked whether expression of LTα in ectopic sites could affect lymphatic vessels. Because, RIPLTα transgenic mice develop cellular accumulations in TLOs in the pancreas, kidney and skin,25 we first evaluated the RIPLTα mouse pancreatic TLOs for lymphatic vessels (Figure 5). Prox1 and LYVE-1 positive vessels were found in the TLOs surrounding the LTα-secreting pancreatic islets (Figure 5A-B). These vessels were more prominent and dense in and around islets of RIPLTα mice than in WT mice (Figure 5B). Morphometric quantification revealed a significantly greater lymphatic vessel density in RIPLTα pancreata compared with those of WT mice (Figure 5C).
Lymphatic vessels associated with tertiary lymphoid organs in the pancreas induced by ectopic expression of LTα. (A) H&E, DAPI, and Prox1 staining of 1-year-old RIPLTα pancreas (40× objective; Zeiss Axioscope). (B) Merge of peri-islet bright field staining for hematoxylin and dark field for LYVE-1 positive lymphatic vessels (red) in the pancreas. More lymphatic vessels are associated with TLOs around the islets of RIPLTα and RIPLTαLTβ−/− mice than WT mice (n = 3 per group; 20× objective; Zeiss Axioscope). (C) Morphometric quantification reveals a significant increase in peri-islet LYVE-1 positive vessel area in the pancreata of RIPLTα and RIPLTαLTβ−/− mice compared with WT mice (*P < .05). No significant difference in LYVE-1 positive vessel area was found between RIPLTα and RIPLTαLTβ−/− pancreata.
Lymphatic vessels associated with tertiary lymphoid organs in the pancreas induced by ectopic expression of LTα. (A) H&E, DAPI, and Prox1 staining of 1-year-old RIPLTα pancreas (40× objective; Zeiss Axioscope). (B) Merge of peri-islet bright field staining for hematoxylin and dark field for LYVE-1 positive lymphatic vessels (red) in the pancreas. More lymphatic vessels are associated with TLOs around the islets of RIPLTα and RIPLTαLTβ−/− mice than WT mice (n = 3 per group; 20× objective; Zeiss Axioscope). (C) Morphometric quantification reveals a significant increase in peri-islet LYVE-1 positive vessel area in the pancreata of RIPLTα and RIPLTαLTβ−/− mice compared with WT mice (*P < .05). No significant difference in LYVE-1 positive vessel area was found between RIPLTα and RIPLTαLTβ−/− pancreata.
To assess whether LTβ was required for the effect on lymphangiogenesis seen in RIPLTα mice, we compared pancreatic lymphatic vessel density in RIPLTαLTβ−/− mice to that of RIPLTα and WT mice. There was no significant difference in lymphatic vessel density between RIPLTα and RIPLTαLTβ−/− mice. In fact, lymphatic vessel density was significantly greater in the pancreata of both strains compared with WT (Figure 5C). This suggested that LTα alone can induce both TLO formation25 and lymphangiogenesis within the TLO in the absence of LTβ.
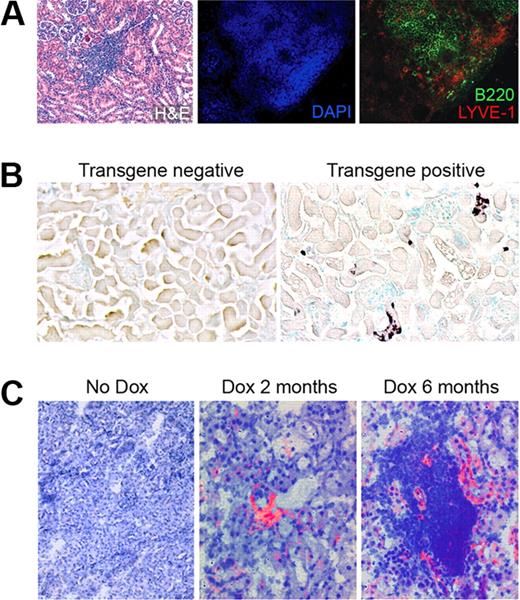
LYVE-1 positive lymphatic vessels were also found in the kidney TLOs of RIPLTα mice in regions that included both B (Figure 6A) and T cells (data not shown). Therefore, we next examined the kinetics of lymphatic vessel increase with regard to LTα expression and the development of cellular accumulations using RIPLTαTetOn mice, in which doxycycline feeding induces LTα expression in the convoluted tubules of the kidney. In these mice, there was a gradual increase in the number of infiltrating cells that paralleled the increasing intensity of LTα expression in the kidney tubules after doxycycline feeding. Thus, this system is an appropriate tool to assess lymphatic vessel development with respect to LTα expression over time. After 4 days of doxycycline feeding, in situ hybridization revealed LTα mRNA expression in the convoluted tubules of the kidney of RIPLTαTetOn mice, as expected from previous observations in RIPLTα mice.45 A gradual increase was apparent, with more cells in the tubules expressing the transgene at even higher levels at later times (Figure 6B).
Lymphatic vessels are apparent before obvious TLOs. (A) H&E, DAPI, B220, and LYVE-1 staining in the kidney of a RIPLTα mouse (40× objective; Zeiss Axioscope). LYVE-1 positive lymphatic vessels (red) are within and around B cell regions (green) of the TLO. (B) In situ hybridization reveals LTα expression (dark purple) in the convoluted tubules of a RIPLTαTetOn mouse kidney after 1 month of doxycycline feeding (20× objective; Zeiss Axioscope). (C) Merge of bright field staining for hematoxylin and dark field for LYVE-1 positive vessels (red) reveals lymphatic vessels within kidney parenchyma after 2 months of doxycycline feeding before apparent extensive leukocytic infiltration. At 6 months, TLOs are apparent in the kidney with more obvious lymphatic vessels Cy-2 green LYVE-1 staining has been digitally colorized red to enhance contrast against hematoxylin (original magnification ×40; Zeiss Axioscope).
Lymphatic vessels are apparent before obvious TLOs. (A) H&E, DAPI, B220, and LYVE-1 staining in the kidney of a RIPLTα mouse (40× objective; Zeiss Axioscope). LYVE-1 positive lymphatic vessels (red) are within and around B cell regions (green) of the TLO. (B) In situ hybridization reveals LTα expression (dark purple) in the convoluted tubules of a RIPLTαTetOn mouse kidney after 1 month of doxycycline feeding (20× objective; Zeiss Axioscope). (C) Merge of bright field staining for hematoxylin and dark field for LYVE-1 positive vessels (red) reveals lymphatic vessels within kidney parenchyma after 2 months of doxycycline feeding before apparent extensive leukocytic infiltration. At 6 months, TLOs are apparent in the kidney with more obvious lymphatic vessels Cy-2 green LYVE-1 staining has been digitally colorized red to enhance contrast against hematoxylin (original magnification ×40; Zeiss Axioscope).
A few LYVE-1 positive vessels were found in the kidney cortex of RIPLTαTetOn transgenic mice within 2 months of doxycycline feeding prior to the development of obvious TLOs. Although it is possible that scattered leukocytes could be present in the kidney, they were not detected at this time (Figure 6C). However, at later times after doxycycline feeding prominent TLOs were evident in the kidney. Lymphatic vessels were found within and around these leukocyte accumulations (Figure 6C) in a distribution similar to that seen in the kidneys of constitutively over expressing RIPLTα mice (Figure 6A). These data suggest that minimal lymphangiogenesis can occur before the development of obvious organized cellular infiltrates, cellular compartmentalization, or HEVs, and suggests that even in the absence of an infiltrate, LTα may induce lymphangiogenesis.
Discussion
The data presented in this study demonstrate that LTα, in addition to its known roles in the development of secondary19 and tertiary lymphoid organs,25 plays a role in both lymphatic vessel function and lymphangiogenesis in both inflammation and the development of TLOs. We show that a deficiency in LTα results in a reduction in lymph flow velocity. This defect appears to be compensated for in LTα−/− mice by an elevation in Pif that counteracts lymphatic dysfunction by increasing the filling pressure of the initial lymphatics and by reducing the filtration from blood capillaries, shown to occur during overhydration. Both of these factors contribute to autoregulation of the EFV42 and thus prevent the development of lymphedema which might otherwise have been the consequence of the lymphatic dysfunction. The exact physiologic effect of LTα on lymphatic vessels which produces this alteration of function remains to be determined. This could be an effect on the smooth muscle cells lining the lymphatics that aid in the propulsion of lymph and/or an effect on recently described junctions between the endothelial cells lining these vessels.43 In addition, it is unclear whether the effect of LTα on lymphangiogenesis is direct or indirect. The production of LTα by lymphoid tissue inducer cells or NK cells may activate stromal cells to produce VEGF-C, a crucial mediator of lymphangiogenesis in ontogeny2 and inflammation and/or VEGF-A that has been implicated in lymphangiogenesis in inflammation.46,47 Furthermore, LTα, which is known to induce chemokines and adhesion molecules, could contribute to the recruitment of other leukocytes, such as macrophages, that produce VEGF-C. TNFα has been shown to up-regulate the expression of VEGF-C by macrophages.48 LTα could have the same effect.
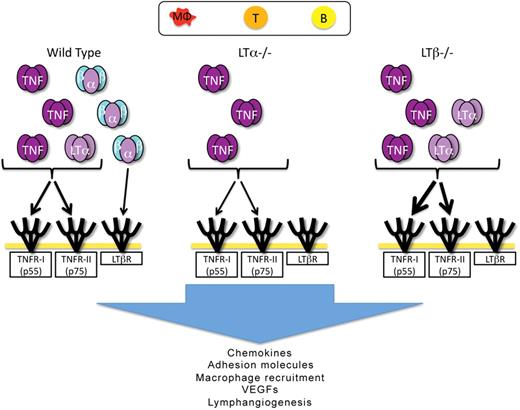
A deficiency in LTβ resulted in more dramatic lymphangiogenesis in the infection and immunization inflammation models studied here. Thus, LTβ does not appear to play a significant positive role in lymphangiogenesis in these models of inflammation, and may actually inhibit this process. We therefore hypothesize that, in the absence of LTβ, LTα monomers are freed to form the LTα3 homotrimer in larger amounts than is possible in the presence of the LTβ monomer, which would otherwise complex the LTα monomers. Thus more LTα3 would be available to signal through the TNFRs on endothelial cells,44 immune cells and stromal cells to produce chemokines. This hypothesis is supported by data indicating that although TNFα plays an important role in the M pulmonis model, inhibition of that molecule does not completely eliminate lymphangiogenesis.44 LTα3 signaling through TNF receptors could, therefore, play a compensatory role. The combination of LTα3 and TNFα3 signaling would thus ultimately lead to lymphangiogenesis, possibly through the mechanisms noted above; ie the induction of growth factors,14 the recruitment of cells that produce these growth factors via the induction of chemokines27,30 and vascular adhesion molecules.25,27,30,45,49 The induction of chemokines and changes in vasculature are likely direct effects of the LT/TNF family that are mediated by the recruitment of inflammatory cells which have been implicated in inducing lymphangiogenesis.10,15,36,47 Figure 7 shows our current working model. Studies to directly test the hypotheses presented here should be possible using recently described reagents that detect secreted mouse LTα3 once they become more widely available.50
Working model of the roles of the LT/TNF family members in lymphangiogenesis in inflammation. The putative cellular origin of cytokines of the LT/TNF family is indicated at the top of the figure. In WT mice, all forms of the cytokines are indicated: TNFα3, LTα3, and LTα1β2. LTα2β1 is not included in the figure. The receptors for the various forms of the cytokines are indicated. LTα−/− mice produce only TNFα3. Even though these mice make LTβ mRNA, no protein is assembled on the cell surface due to the requirement of LTα for the cell surface expression of LTβ. TNFα3 and LTα3 are produced in LTβ−/− mice. The absence of LTβ allows more LTα to assemble as the LTα3 homotrimer, and is an explanation for the more intense inflammation in the absence of LTβ. TNFα3 and LTα3, working through the TNF receptors induce chemokines and inflammatory vascular adhesion molecules that then induce the accumulation of additional cells that can produce factors, such as VEGFs capable of inducing lymphangiogenesis. It is also possible that LTα3 and TNFα3 induce VEGFs directly from stromal cells and could also have direct effects on lymphatic endothelial cells.
Working model of the roles of the LT/TNF family members in lymphangiogenesis in inflammation. The putative cellular origin of cytokines of the LT/TNF family is indicated at the top of the figure. In WT mice, all forms of the cytokines are indicated: TNFα3, LTα3, and LTα1β2. LTα2β1 is not included in the figure. The receptors for the various forms of the cytokines are indicated. LTα−/− mice produce only TNFα3. Even though these mice make LTβ mRNA, no protein is assembled on the cell surface due to the requirement of LTα for the cell surface expression of LTβ. TNFα3 and LTα3 are produced in LTβ−/− mice. The absence of LTβ allows more LTα to assemble as the LTα3 homotrimer, and is an explanation for the more intense inflammation in the absence of LTβ. TNFα3 and LTα3, working through the TNF receptors induce chemokines and inflammatory vascular adhesion molecules that then induce the accumulation of additional cells that can produce factors, such as VEGFs capable of inducing lymphangiogenesis. It is also possible that LTα3 and TNFα3 induce VEGFs directly from stromal cells and could also have direct effects on lymphatic endothelial cells.
When LTα was overexpressed as a transgene, lymphatic vessels were induced in mice deficient in LTβ. Thus, there appears to be no essential requirement for the LTα1β2 complex in this process. This suggests that LTα-induced lymphangiogenesis is mediated by LTα3 signaling through the TNFR1 receptor, as previously reported for the induction of leukocytic infiltrates in RIPLTα kidneys.26 When LTα expression was switched on in the RIPLTαTetOn kidneys, the formation of a small number of lymphatic vessels was apparent prior to the organization of large distinct leukocytic infiltrates in the TLO. Nevertheless, it is possible that small numbers of infiltrating leukocytes were already present and contributed to this effect. Although previous work has established a role for LTβR in lymphangiogenesis in thyroid TLOs,15 our data demonstrate that LTα alone can induce lymphangiogenesis within pancreas and kidney TLOs. LTβ may, therefore, play different roles in lymphangiogenesis in different models of inflammation.
The roles of LTs in inflammation and lymphoid organ development have been known for many years. The data presented here support an additional role—that of lymphangiogenesis. We have probed the roles of the individual LT members and conclude that LTα contributes to lymphatic vessel function in steady state conditions and induces lymphangiogenesis in inflammation. It is likely that additional cytokines also contribute to these processes, and that TNFα is a probable candidate in inflammation. Although LTβ has been shown to play a role in lymphangiogenesis in the thyroid undergoing transgene-induced chronic inflammation,15 it appears not to be essential for lymphangiogenesis in the TLO models studied here, and could even impede the process in inflammation.
Lymphatic vessels play crucial roles in fluid balance, immune responses, and tumor metastases and are defective or absent in hereditary and surgically induced lymphedema. The data provided here contribute to our understanding of lymphangiogenesis and will provide insight into potential therapeutic manipulations of this process.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Myriam Hill for excellent technical assistance and image preparation, Drs Prabir Ray, Carolyn Cuff, and Rosalba Sacca for assistance in the preparation and initial analysis of the RIPLTαTetOn mice, Carl Erik Markhus for assistance in the colloid osmotic pressure measurements, Tarek Fahmy for preparation of the fluorescein conjugated nanoparticles, and Eitan Akirav for helpful discussion
This work was supported by National Institutes of Health (NIH) R01DK 57731 and CA 16885 (N.H.R.); NIH P01 HL24136, R01HL59157, and R01CA82923 (D.M.M.); NIH R01CA085149 (R.K.J.); NIH K99CA137167 (T.P.P.), a pilot grant from NIH P30 041942 (N.H.R.), a Fellowship from the Lymphatic Research Foundation (R.H.M.), Western Norway Regional Health Authority (O.S.S.), and The Research Council of Norway and the Norwegian Cancer Society (H.W.).
National Institutes of Health
Authorship
Contribution: R.H.M. designed and conducted research, analyzed and interpreted data, and wrote the manuscript; O.S.S. designed and conducted research and analyzed and interpreted data; P.B. designed and conducted research, analyzed and interpreted data, and wrote the manuscript; C.M.B. conducted research and analyzed and interpreted data; T.P.P. designed and conducted research, analyzed and interpreted data, and wrote the manuscript; H.W. designed research, analyzed and interpreted data, and wrote the manuscript; R.K.J. designed research and analyzed and interpreted data; D.M.M. designed research and analyzed and interpreted data; and N.H.R. designed and conducted research, analyzed and interpreted data, and wrote the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
The current affiliation for R.H.M. is University of Pittsburgh Medical Center Montefiore Hospital, Department of Internal Medicine, Pittsburgh, PA.
Correspondence: Nancy H. Ruddle, PhD, 10 Amistad St, Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT 06520; e-mail: nancy.ruddle@yale.edu.

![Figure 1. Analysis of lymph flow velocity and physiology. (A) Quantitative analysis using residence time distribution theory reveals a significant difference in the lymph flow velocity of fluorescent dextran in tail lymphatics between LTα−/− and wild type (WT) mice, but not between LTβ−/− and WT mice (a minimum of n = 10 mice per group; *P < .05). (B) Extracellular fluid volume (EFV) in paw skin measured during steady state control situation () and after overhydration (). In contrast to LTα−/− mice (n = 8 in control [c] and overhydration [o]), both LTβ−/− (n = 8 [c] and n = 9 [o]) and WT (n = 6 [c] and n = 4 [o]) mice show a significant increase in EFV after overhydration (*P < .05). (C) LTα−/− mice (n = 16) have a significantly higher interstitial fluid pressure (Pif) than both LTβ−/− (n = 17) and WT mice (n = 10) during steady state control situation (*P < .05). All 3 groups, however, demonstrate a significant increase in Pif after overhydration (#P < .05). (D) Colloid osmotic pressure (COP) in plasma and interstitial fluid in the steady state control situation. LTα−/− mice show comparable COP in the interstitial fluid to WT and LTβ−/− mice. (E) After overhydration, the LTα−/− mice (n = 6) show a significantly higher COP in the interstitial fluid in comparison to WT mice (n = 7; *P < .05). (F) Colloid osmotic pressure gradient (ΔCOP) across the capillaries in the steady state control situation and after overhydration. All 3 groups show a nonsignificant decrease in ΔCOP after overhydration. Overhydrated LTα−/− (n = 6) mice have a significantly lower ΔCOP compared with WT (n = 7) mice (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/12/10.1182_blood-2009-12-256065/4/m_zh89991057730001.jpeg?Expires=1769718371&Signature=qtkgroK1JTg4GsrCOC4kcTrmF41GGf0FbjG8e8-OnVqU2P5kxQsIrUorKTSH-IYReKpgsm6l-I2ePKEH52AaCnLYt-okKEufIJlvIRgV7XUhZysNRzpZD4gTEO1-zIlmXzFDBaPnAIJ5blS9ndXLdZt~v1jyoeBXoGTwDJpQ9MArvFXBXMzFMhf~i8jo1VsKEthWJebwuzLn0dAwXcK8lyTAt8355MgRHIHAUH7ucBJqAHlLW4zmnx2GTPVGe6n~6oMLgdpptKshOCRYry1lOEu-EUniLjzilJQ9SzmyLzseW8xEJiTH7hYMf~gxz1ePvPndrfsYQ3KWb-hYjFlEYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 1. Analysis of lymph flow velocity and physiology. (A) Quantitative analysis using residence time distribution theory reveals a significant difference in the lymph flow velocity of fluorescent dextran in tail lymphatics between LTα−/− and wild type (WT) mice, but not between LTβ−/− and WT mice (a minimum of n = 10 mice per group; *P < .05). (B) Extracellular fluid volume (EFV) in paw skin measured during steady state control situation () and after overhydration (). In contrast to LTα−/− mice (n = 8 in control [c] and overhydration [o]), both LTβ−/− (n = 8 [c] and n = 9 [o]) and WT (n = 6 [c] and n = 4 [o]) mice show a significant increase in EFV after overhydration (*P < .05). (C) LTα−/− mice (n = 16) have a significantly higher interstitial fluid pressure (Pif) than both LTβ−/− (n = 17) and WT mice (n = 10) during steady state control situation (*P < .05). All 3 groups, however, demonstrate a significant increase in Pif after overhydration (#P < .05). (D) Colloid osmotic pressure (COP) in plasma and interstitial fluid in the steady state control situation. LTα−/− mice show comparable COP in the interstitial fluid to WT and LTβ−/− mice. (E) After overhydration, the LTα−/− mice (n = 6) show a significantly higher COP in the interstitial fluid in comparison to WT mice (n = 7; *P < .05). (F) Colloid osmotic pressure gradient (ΔCOP) across the capillaries in the steady state control situation and after overhydration. All 3 groups show a nonsignificant decrease in ΔCOP after overhydration. Overhydrated LTα−/− (n = 6) mice have a significantly lower ΔCOP compared with WT (n = 7) mice (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/12/10.1182_blood-2009-12-256065/4/m_zh89991057730001.jpeg?Expires=1769718372&Signature=kIerWNlCaTdOOLIHMrJ6mF2tgh4PyM5Ca~E-q2CJXSzK8THcXLWSPvtZhBdKTcnHFKpqb8GEtQVCX-oxXzZYn4MnaQ62MLbqRBzQ1CKDS22VDmncqn6WxtbBhCi5xFl0K1ch~vCspeTVNy2ye72qSSP9b3JCYzipWWRx1aYQ7hDCFmyZcUVMsq1lyHOjjgKuNdG4OTsHhMHG1MKXo1EPHAe~HTIUjy5LTGbSLHAF9XEjJtRGEJlWaT4H7i1Q81iZZWgjQBH-rIaarlc5auX~XI9ZF5ib7LzFBaIi-cB~XDnl-pFBldVKwjINOsPj4erA9D7JohukJrcA5-9RUmyMWQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
 ) and after overhydration (
) and after overhydration ( ). In contrast to LTα−/− mice (n = 8 in control [c] and overhydration [o]), both LTβ−/− (n = 8 [c] and n = 9 [o]) and WT (n = 6 [c] and n = 4 [o]) mice show a significant increase in EFV after overhydration (*P < .05). (C) LTα−/− mice (n = 16) have a significantly higher interstitial fluid pressure (Pif) than both LTβ−/− (n = 17) and WT mice (n = 10) during steady state control situation (*P < .05). All 3 groups, however, demonstrate a significant increase in Pif after overhydration (#P < .05). (D) Colloid osmotic pressure (COP) in plasma and interstitial fluid in the steady state control situation. LTα−/− mice show comparable COP in the interstitial fluid to WT and LTβ−/− mice. (E) After overhydration, the LTα−/− mice (n = 6) show a significantly higher COP in the interstitial fluid in comparison to WT mice (n = 7; *P < .05). (F) Colloid osmotic pressure gradient (ΔCOP) across the capillaries in the steady state control situation and after overhydration. All 3 groups show a nonsignificant decrease in ΔCOP after overhydration. Overhydrated LTα−/− (n = 6) mice have a significantly lower ΔCOP compared with WT (n = 7) mice (*P < .05).
). In contrast to LTα−/− mice (n = 8 in control [c] and overhydration [o]), both LTβ−/− (n = 8 [c] and n = 9 [o]) and WT (n = 6 [c] and n = 4 [o]) mice show a significant increase in EFV after overhydration (*P < .05). (C) LTα−/− mice (n = 16) have a significantly higher interstitial fluid pressure (Pif) than both LTβ−/− (n = 17) and WT mice (n = 10) during steady state control situation (*P < .05). All 3 groups, however, demonstrate a significant increase in Pif after overhydration (#P < .05). (D) Colloid osmotic pressure (COP) in plasma and interstitial fluid in the steady state control situation. LTα−/− mice show comparable COP in the interstitial fluid to WT and LTβ−/− mice. (E) After overhydration, the LTα−/− mice (n = 6) show a significantly higher COP in the interstitial fluid in comparison to WT mice (n = 7; *P < .05). (F) Colloid osmotic pressure gradient (ΔCOP) across the capillaries in the steady state control situation and after overhydration. All 3 groups show a nonsignificant decrease in ΔCOP after overhydration. Overhydrated LTα−/− (n = 6) mice have a significantly lower ΔCOP compared with WT (n = 7) mice (*P < .05).