Abstract
The aim of this study was to determine the efficacy and safety of treatment of pediatric acute promyelocytic leukemia (APL) with single-agent arsenic trioxide (ATO). A total of 19 children (≤ 15 years of age) with newly diagnosed APL were treated with single-agent ATO for remission induction and postremission therapy. Seventeen of the children (89.5%) achieved complete hematologic remission, and 2 early deaths occurred from intracranial hemorrhage. ATO-induced leukocytosis was observed in 13 (68.4%) patients. Other ATO-related toxicities were minimal and transient. Postremission ATO therapy continued for 3 years; the most common side effect was ATO-induced neutropenia. With a median follow-up of 53 months (range, 23-76 months), the calculated 5-year overall survival and event-free survival were 83.9% and 72.7%, respectively, which are comparable with results achieved by the use of ATRA plus chemotherapy, which is the standard therapy for APL. No chronic arsenic toxicity or second malignancies were found during the follow-up period, and arsenic retention was not significant in patients off treatment more than 24 months. ATO resistance was observed in only 1 patient with a complex karyotype. The results indicate the high efficacy and safety of single-agent ATO regimens in the treatment of children with de novo APL.
Introduction
Acute promyelocytic leukemia (APL) accounts for approximately 10% of childhood acute myeloid leukemia.1-4 APL is characterized by the French-American-British M3 subtype morphology, a distinctive immunophenotype, and, in the majority of cases, there is a t(15;17) chromosomal translocation that generates the promyelocytic leukemia–retinoic acid receptor-α (PML-RARa) fusion gene. This specific genetic lesion determines the unique response to treatment with all-trans retinoic acid (ATRA) or arsenic trioxide (ATO).5
Since the introduction of ATRA in the 1980s and ATO in the 1990s, the strategy for treating APL has shifted from conventional chemotherapy to cancer cell differentiation and cancer-targeted therapy. The combination of ATRA and anthracycline-based chemotherapy is currently the standard approach to treat newly diagnosed APL. With this regimen, the hematologic complete remission (HCR) rate has improved significantly, to more than 90%; 5-year disease-free survival is more than 70%.5-10 However, some problems still remain; notably, ATRA-related side effects appear to be more pronounced in children than in adult patients,4,11-13 the optimal postremission therapy still remains to be defined, and approximately 20% to 30% of patients eventually relapse and develop drug resistance.
ATO has proven to be another highly effective agent in APL therapy.14-21 ATO differs from ATRA because of its dual effects of inducing partial differentiation and apoptosis of APL cells. Although highly efficacious, ATO is most commonly used in refractory or relapsed APL for inducing remission. It has previously been shown that single-agent ATO is equally effective in inducing remission in newly diagnosed cases of adult APL. However, little is known about the use of single-agent ATO in the treatment of children with APL. The optimal dosage and route of administration during remission induction and postremission therapy, the HCR rate and long-term efficacy, and, most importantly, ATO-induced toxicity and long-term safety have yet to be determined.
It was in our hospital that ATO initially was used with success in treating adult APL.14 We have since modified the ATO protocol for childhood APL. Here, we report our experience with ATO as a single agent for remission induction and postremission therapy for children with newly diagnosed APL.
Methods
Patients
The study was open to children with newly diagnosed APL who were being treated in the First Affiliated Hospital of Harbin Medical University from August 2002 to January 2007. The study protocol was reviewed and approved by the Heilongjiang Provincial Medical Ethics Committee. During this period, a total of 28 children younger than 16 years of age were diagnosed with APL. Of these patients, 9 (6 female, 3 male) did not receive any treatment after diagnosis for personal economic reasons. In accordance with the Declaration of Helsinki informed consent was obtained from guardians of 19 child patients, and they were treated with the ATO protocol. All patients were diagnosed with APL on the basis of the characteristic French-American-British M3 bone marrow morphology and peripheral blood leukemia cells. Confirmation was obtained with bone marrow mononuclear cells by the use of conventional cytogenetics, showing t(15;17) and/or by positive reverse transcription polymerase chain reaction (RT-PCR) assay or fluorescence in situ hybridization tests for PML/RARa fusion.
Induction therapy protocol
ATO solution (10 mg/10mL) was supplied by the Harbin Yida Pharmaceutical Company. Intravenous ATO infusion, prepared in 5% dextrose, was administered daily at the dose of 0.20 mg/kg for children 4 to 6 years of age and 0.16 mg/kg for those older than 6 years of age, with a maximum daily dose of 10 mg. The total daily dose was infused intravenously over the course of 2 to 4 hours. ATO infusion was given daily until achievement of HCR or to a maximum of 60 doses.
Postremission therapy
Postremission therapy continued for 3 years. Usually, ATO was administered daily for a total of 14 doses per course, and daily dose was modulated according to age and body weight. A 14-day course was repeated with 4-week intervals during the first year after HCR. The interval between courses was increased to 2 months during the second year and 3 months during the third year. But the therapeutic regimen would be adjusted on the basis of specific conditions. If one of the following events occurred, that is, the PML/RARa fusion gene reappeared, the blasts plus promyelocytes in bone marrow increased to 3.5% to 5%, or the white blood cell (WBC) count did not decrease markedly after 14 days of ATO administration compared with that before the ATO administration, the duration of 1 cycle would be extended to 21 to 28 days, and the interval would be suitably shortened. From our experience, patients under the aforementioned conditions were at risk of relapse.
Patients received postremission therapy as outpatients. Prophylactic intrathecal injection of methotrexate 4 to 5 mg, Ara-C 10 to 15 mg, and dexamethasone 2 to 3 mg was given once or twice a week up to 6 times after remission. Commonly when the first cycle of postremission therapy was initiated, prophylactic intrathecal injections were begun at the same time. Cerebrospinal fluid was simultaneously obtained and routine and biochemical analysis were performed.
Monitoring
During induction therapy, complete blood count (CBC) results were observed every other day. Coagulation parameters, including prothrombin time, activated partial thromboplastin time, fibrinogen, and D-dimer were observed every other day during coagulopathy. Liver functions, renal functions, and electrolytes were tested twice weekly. Electrocardiograms were required weekly. Bone marrow examination was performed 2 to 3 weeks after therapy started and was repeated weekly until HCR was confirmed.
In patients who did not achieve molecular complete remission (MCR) immediately after HCR, RT-PCR assays for the PML/RARa fusion transcript with bone marrow specimens were done every 3 months, before or after a certain course of postremission therapy (not after each course of therapy), until MCR was achieved. For patients who achieved MCR, molecular monitoring was done every 3 to 6 months for 5 years after HCR, afterward, once a year for 3 years. For every ATO course during postremission therapy, CBCs were done before the administration of ATO and were repeated once a week thereafter.
Throughout postremission therapy and for at least 3 years after completion of all therapy, complete physical examinations and the following tests were performed every 3 to 6 months: liver function, renal function, electrolytes, electrocardiograms, dermatologic consultations, and detailed neurologic examinations.
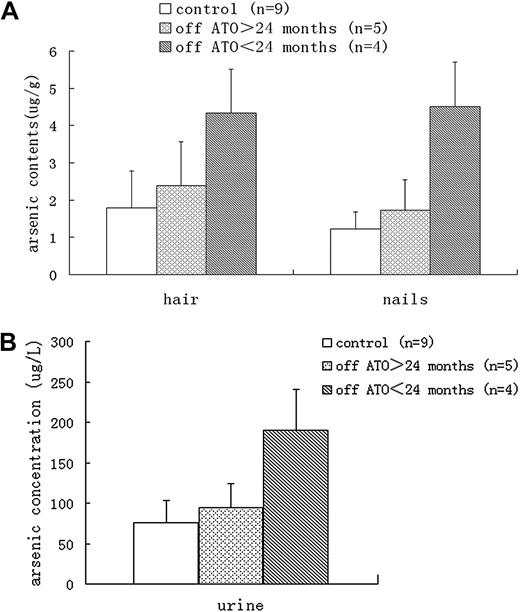
The arsenic concentrations in urine samples and the arsenic content in nails and scalp hair were measured in 9 patients 10 to 38 months after treatment ended. Arsenic concentrations were determined by hydride generation atomic fluorescence spectrometry. The collected specimens were placed into polypropylene tubes. Urine specimens were stored at 4°C and were analyzed within 2 weeks. For each assay, 5.0 mL of urine or 1 g of nails or hair was needed. For wet digestion, samples were digested with repeated additions of nitric acid, sulfuric acid, and perchloric acid and heated on an electric hot plate. Mixtures were then treated with thiocarbamate to ensure quinquevalent arsenic was reduced to trivalent arsenic. Arsenic fluorescent intensity data were assayed by AFS-9800 double-channel atom fluorophotometer (Haiguang Equipment Company). Arsenic concentrations were obtained with the standard-curve method (linear range, 0-200 ng/mL). The detection limit of the method was 0.01 μg/L.
Supportive therapy
Platelet transfusions were given to maintain platelet counts greater than 20 × 109/L. Fresh-frozen plasma (15 mL/kg per day) was infused repeatedly when there were indications of coagulopathy or clinical indications of active bleeding. If fibrinogen remained less than 0.8 to 1 g/L after multiple infusions of fresh-frozen plasma, then cryoprecipitate was administered to increase fibrinogen levels. Intravenous fluids and supplemental electrolytes were administered to maintain levels within the normal range; in particular, the potassium level was kept greater than 4 mEq/dL, magnesium was kept greater than 1.8 mg/dL, and the serum calcium level was kept within the normal range (2.25-2.74mM). Antibiotics were administered as required for controlling infections and fevers. When total WBC count was 20 × 109/L or greater, hydroxyurea (HU; 1.0-2.0 g) was administered to patients orally each day, in 2 or 3 divided doses. During the period of HU use, the CBC was closely monitored (tested once a day), and as soon as the WBC count decreased to 20 × 109/L or less, HU was discontinued. When WBC count was greater than 20 × 109/L, NaHCO3 and allopurinol were commonly administered orally, and no extra hydration was needed. If patients developed APL differentiation-like syndrome, dexamethasone (5 mg twice daily) was used for at least 3 days, and, if necessary, ATO was discontinued temporarily.
Outcome definitions
HCR was defined as the presence of all of the following: no clinical evidence of APL, peripheral-blood neutrophil count 1.5 × 109/L or greater, platelet count 100 × 109/L or greater, no blasts or promyelocytes in blood, and less than 5% blasts plus promyelocytes in the bone marrow. Hematologic relapse was defined as the recurrence of leukemic blasts in blood or as the recurrence greater than 5% blasts plus promyelocytes in the bone marrow after a HCR was achieved. MCR was defined as negative RT-PCR test results obtained after HCR in patients with a positive RT-PCR test at diagnosis.
Early death was defined as death within the first 2 weeks of induction treatment. Overall survival (OS) was calculated as the time from the initiation of ATO treatment to death, and patients still alive were censored at the time of last contact. Event-free survival (EFS) was calculated as the time from the first day of therapy to death or relapse. In the absence of these events, patients were censored at the last visit.
Statistical methods
Patient characteristics were evaluated by descriptive statistics. OS and EFS were derived by the use of the Kaplan-Meier method. A 1-way analysis of variance, followed by a Dunnett test, was used to compare arsenic levels in treated groups with those in the healthy control group.
Results
Remission induction
A total of 19 patients, 4 to 15 years of age, were treated with ATO and included in this study. The clinical and hematologic features at diagnosis are summarized in Table 1. Karyotyping was performed in 11 cases at diagnosis; 10 of them had t(15;17), and 1 did not. In addition, a complex karyotype, 46,XY,t(15;17)[3]/46,XY,t(15;17),+8, −19[1]/47,XY,t(15;17),+8[17], was observed in patient 19.
Of the 19 patients, 17 (89.5%) achieved HCR with the single-agent ATO regimen. The median duration required to achieve HCR was 38 days (range, 26-55 days). The median cumulative dose of ATO for HCR was 6.1 mg/kg (range, 3.68-10.8 mg/kg).
The failure to achieve HCR in 2 patients was attributed to early death. They both had the M3a of APL and died on day 6 of ATO therapy (Table 1). It is worth noting that these 2 patients presented with the 2 highest leukocyte counts at diagnosis (patients 4 and 9; Table 1), and both had severe leukocytosis. Their WBC counts increased dramatically in response to ATO treatment, from 38.6 × 109/L and 89.6 × 109/L to 177.7 × 109/L and 251.7 × 109/L in 4 to 6 days, respectively. Their deaths were presumably from intracranial hemorrhage. Computed tomography and magnetic resonance imaging on these patients were not obtained because in both patients the symptoms of increased intracranial pressure appeared suddenly and the disease progression was so rapid that they became unconscious shortly thereafter. Considering that patients with intracranial hemorrhage are not suitable to be moved, we had planned to perform magnetic resonance imaging scans on them as soon as their conditions became stable. However, they died 2.5 and 7 hours later, respectively, from the appearance of symptoms of intracranial hyperpressure.
According to RT-PCR measurements, with a sensitivity of 10−4, 9 of the 17 patients achieved HCR and had molecular monitoring of PML/RARa fusion. One patient achieved MCR immediately after HCR, and 8 others did so within 3 to 9 months after HCR (median, 3 months). No isolated molecular relapse (not leading to hematologic relapses) occurred during the follow-up period.
ATO-induced leukocytosis and toxicity during induction
An increase in leukocyte counts occurred in all 19 patients during ATO induction. Thirteen patients (68.4%) developed marked leukocytosis, with a total WBC count ranging from 21.9 × 109/L to 251.71 × 109/L (median, 34.7 × 109/L). The median duration of leukocytosis was 16 days (range, 8-25 days). In 3 patients, the ATO dose was reduced as the result of significant leukocytosis. Two patients developed differentiation syndrome–like respiratory distress. They were treated successfully by controlling progressive leukocytosis with the use of hydroxyurea, temporary discontinuation of ATO, and the administration of dexamethasone for 3 to 5 days.
Other side effects of ATO included asymptomatic QTc prolongation (1 of 19), headache (3 of 19), skin rash (2 of 19), facial edema (1 of 19), peripheral neuropathy (1 of 19), musculoskeletal pain (3 of 19), hepatic toxicity (3 of 19), and dryness of the mouth (2 of 19). The headache and hepatic toxicity were grade 1 or 2, according to National Cancer Institute common toxicity criteria. All nonhematologic toxicities were mild and resolved quickly on discontinuation of ATO.
Postremission therapy and tolerance
Sixteen patients who achieved HCR proceeded with postremission therapy, except 1 patient, who was lost to follow-up (patient 10). Postremission therapy continued for 3 years in this study. The periodic ATO infusion regimen was well tolerated. The most common side effect was ATO-induced neutropenia. All 16 patients developed grade 1 neutropenia, but no febrile neutropenia occurred. Other side effects included grade 1 headache (4 patients), skin rash (3 patients), and peripheral neuropathy (2 patients). All the toxicity was reversible and needed no management.
Outcomes and long-term toxicity
The study included follow-up data until December 2008. Of the 16 patients who achieved HCR and received postremission therapy, 13 remained in first HCR for a period of 21 to 75 months (Table 1). Three patients had hematologic relapse. Two of the relapses occurred 14 and 56 months after HCR, respectively. A second HCR was achieved in both, after repeated ATO induction therapy. These 2 patients have remained in second HCR for 53 and 14 months, respectively. The third relapse (patient 19) occurred 17 months after HCR, and failed to respond to repeated ATO induction. Subsequently, the patient was administered ATRA in combination with chemotherapy and achieved a second HCR. However, the patient died in a second relapse that occurred 7 months later. It is worth noting that this patient was the case with a complex karyotype. Overall, with a median follow-up duration of 53 months (range, 23-76 months), the Kaplan-Meier estimates of 5-year OS and EFS were 83.9% (95% confidence interval, 67.2%-100%) and 72.7% (95% confidence interval, 52.3%-93.2%), respectively (Figure 1).
The Kaplan-Meier OS and EFS curves for 19 children treated with ATO. + indicates censoring time.
The Kaplan-Meier OS and EFS curves for 19 children treated with ATO. + indicates censoring time.
One issue of concern was the long-term toxicity induced by exposure to multiple cycles of ATO. Careful evaluation showed that until the final follow-up, all of the 15 patients remaining in HCR were in generally good health and that neither chronic arsenic intoxication (including neurologic toxicity) nor second malignancy had been observed.
The other major concern about the use of ATO was arsenic retention. Urine arsenic concentrations and arsenic contents in nails and hair, which are good indicators of long-term exposure and in vivo accumulation of arsenic, were analyzed in 9 patients at the last follow-up visit, when all therapy had been completed 10 to 38 months previously. Although the arsenic levels in urine, hair, and nails from patients who had ceased treatment for less than 24 months were significantly greater than those in healthy controls (patients' parents; Purine = .000, Phair = .003, Pnails = .000), no significant difference was found between patients who had ceased treatment for more than 24 months and healthy controls (Purine = .556, Phair = .542, Pnails = .436; Figure 2). The urine arsenic concentrations in patients who had ceased treatment for more than 24 months were all below the safe limit of 200 μg/L set by the US Agency for Toxic Substances and Disease Registry (ARTSDR).
Arsenic levels. In hair, nails, (A) and urine (B) samples from different groups.
Arsenic levels. In hair, nails, (A) and urine (B) samples from different groups.
Discussion
This study presents a small but significant series that used a single-agent ATO regimen for both remission induction and postremission therapy of children with newly diagnosed APL. A similar study by George et al22 was conducted in small series of patients, and we summarize the main results from that study in comparison with ours in Table 2. In both studies, with a similar HCR rate of approximately 90%, no patient failed to respond to ATO induction. The only reason for failure to achieve HCR was early death, with 2 early deaths among 19 patients in our study and 1 early death among 11 in the study by George et al.22 Considering the abrupt onset and rapidly deterioration of the 2 catastrophic events, we speculate that the 2 sudden deaths in our study were most likely secondary to uncontrollable intracranial hemorrhage, although the possibility of fatal hemorrhagic infarction of the brain induced by severe leukocytosis and hyperviscosity cannot be excluded in the absence of imaging studies. In the study by George et al,22 single-agent ATO also was used for postremission therapy. Although the postremission therapy was less intensive than ours, and the estimated 5-year OS and EFS were greater than ours, this difference can be attributed to the small sample sizes in both studies and another speculative factor, the different ethnicities of the study participants who had various genetic backgrounds and distinct reactivities to ATO.
Similar to the results in adult patients, the combination of ATRA and intensive chemotherapy has been proven effective in children with APL.2-4 When single-agent ATO is used for remission induction and postremission therapy in children with newly diagnosed APL, it appears to achieve an HCR rate and durable remission comparable with those obtained by use of the combination of ATRA and chemotherapy (Table 2).
Regardless of the presenting leukocyte counts, leukocytosis occurred in response to ATO during remission induction. This finding is consistent with the ATO effect inducing APL differentiation. An increase in leukocyte count was observed in all patients in this study. Severe leukocytosis with a WBC count of greater than 100 × 109/L was observed in 5 of the 19 patients, including 2 early deaths and 2 other patients who developed differentiation-like syndrome. It is interesting to note that, in contrast to leukocytic response to ATO induction, postremission ATO therapy invariably induced neutropenia in patients in HCR. This finding likely reflects the differential effects of ATO on APL cells and maturing granulocytes. ATO apparently induces differentiation of APL cell by degrading the PML-RARa fusion transcript. In addition, it also activates the apoptotic cascade. ATO-induced neutropenia in HCR patients was likely attributed to its apoptotic effect on maturing cells and possibly normal granulocytes.
Of the 3 relapsed patients, a second HCR was achieved in 2 after repeat ATO induction therapy and remained for 14 and 53 months, respectively. This result suggests that the single-agent ATO regimen for APL may not increase ATO-resistance rates.
It is worth mentioning that a greater dose of ATO (0.20 mg/kg) was used in younger patients (4-6 years of age) in this study. In 1970s, when ATO was first used for clinical treatment of APL in our hospital, the dose for younger patients was not defined, and back then no report could provide a reference. Considering that a faster metabolism in children probably promotes drug excretion, we attempted to administer them a little greater dose of ATO, just as the usage of some antibiotics. During treatment toxic response to ATO was observed carefully. Although a greater dose of ATO was used as a single-agent for induction therapy, it caused only minimal toxicity except severe leukocytosis in our patients, as in adults.15,16 Pharmacokinetics study of ATO in children was requested urgently, but because of the need for multiple blood samples and bleeding diathesis in patients, it was always refused by children's parents.
Another important feature of ATO therapy in this study is that the postremission therapy lasts for 3 years. The European researchers have been using 2 years, whereas the North American researchers have been using only 1 year of “maintenance” therapy for APL in children.2-4,17 According to our previous experience, HCR rate was mainly influenced by remission induction therapy, whereas the relapse rate was highly correlated with postremission therapy. By careful observation we have found that when the postremission therapy continued for no more than 2 years here, approximately one-half of the patients (including adults) eventually relapsed 1 to 3 years after the treatment had ended. Prolonging the duration of postremission therapy to 3 years could remarkably decrease the relapse rate. Of course, extending the duration of postremission therapy to reduce potential relapse risk has to be at the expense of increasing the potential risk of cumulative toxicities.
Therefore, a matter of great concern is whether long-term postremission therapy for children with ATO can result in chronic arsenic toxicity, such as skin lesions (pigmentation and keratosis), hypertension, cardiovascular diseases, diabetes mellitus, neurologic effects, cancer of skin, lung, and urinary bladder.23 The present study shows that although periodic ATO therapy continued for more than 3 years, no severe side effects were documented, nor was a second malignancy encountered, with follow-up to 3 or more years after completion of therapy. The analysis of arsenic levels in urine, nails, and hair from patients indicated that no significant ATO accumulation was observed in patients who had been off ATO therapy for more than 2 years.
Decreased hospital time and decreased medical care expenses are the other benefits of our treatment approach. The absence of the toxicities of conventional chemotherapy and of hospitalization during postremission therapy make the ATO treatment regimen less expensive and more acceptable than the ATRA plus chemotherapy regimen in our setting, especially for pediatric patients.
In conclusion, the present study provides additional information on the use of single-agent ATO for induction and postremission therapy in childhood APL. Our results showed that the therapeutic efficacy of the ATO treatment regimen is comparable with that of ATRA plus chemotherapy. Furthermore, this regimen is safe, with minimal toxicity and without significant risk for the development of chronic arsenic toxicity or second malignancy. The advantages of this regimen are that one can use it instead of chemotherapy in children and that it seldom causes drug resistance; these properties make ATO a particularly attractive therapeutic regime. Further investigation with larger sample sizes and risk-adapted postremission therapy are needed.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs M. Minden, D. Amato, and C. Wang of the University of Toronto for their critical reading and editing of the manuscript and Dr Shibo Li of the University of Oklahoma Health Science Center for his help on fluorescence in situ hybridization tests.
Authorship
Contribution: J.Z. designed the research; J.L., X. Li, X.H., L.H., S.W., Y. Zhao, Ying Zhang, S.F., C.L., and L.L. performed the research; Y. Zhao, L.Z., and X. Liu analyzed the data; Yingmei Zhang wrote the paper; and X. Li and J.H. revised the manuscript and answered reviewers' questions.
Conflict-of-interest-disclosure: The authors declare no competing financial interests.
Correspondence: Zhou Jin, Department of Hematology, First Affiliated Hospital, Harbin Medical University, Harbin, Heilongjiang 150001, China; e-mail: zhoujin1111@126.com.