In this issue of Blood, Buil and colleagues combine data from genome-wide associations, a case-control study, and expression and plasma analyses to implicate unbound C4BP, but not protein S, as a risk factor for venous thrombosis (see figure). Their study provides a new basic research direction into the functions of unbound C4BP not involving protein S.
Buil and colleagues, in this issue of Blood, fine map a new susceptibility locus for venous thrombosis to 1q32, at the position where the C4BPA and C4BPB genes are located.1 Although this feat falls short of the conclusive identification of causative single nucleotide polymorphisms (SNPs),2 a strength of the study lies in the efforts to explore the contribution of candidate SNPs to venous thrombosis. C4b-complementary binding protein (C4BP) circulates in the blood in the different isoforms comprising α and β subunits. The investigators establish that increased C4BPA expression in monocytes was associated with SNPs at the locus, and that plasma levels of the unbound α7β0 isoform were elevated in venous thrombosis patients. Their combination of genetic approaches and validation experiments unexpectedly point to the importance of the α7β0 isoform, which is the only circulating form of C4BP unable to bind protein S.
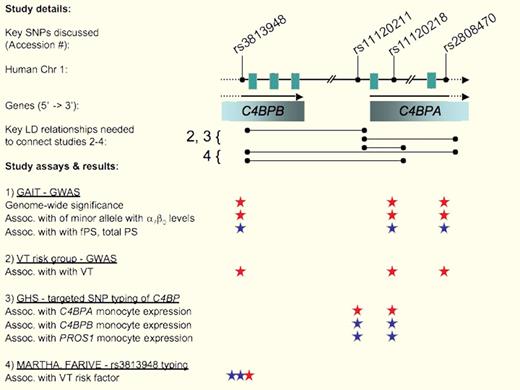
The complement binding protein gene cluster containing C4BPB and C4BPA mapped as new genetic risk factor for venous thrombosis (VT) by means of genome-wide association mapping studies (GWAS) in the GAIT cohort (1), a VT-risk group (2), targeted SNP typing in the GHS study (3), single SNP case-control analyses (rs3813948) of the MARTHA and FARIVE cohorts (4). Key linkage disequilibrium (LD) relationships between key SNPs employed by Buil et al in the context of studies. Significant support and key nonsignificance of assays are shown as red and blue, respectively. Significance was observed only for a combined analysis of both cohorts, but not for each cohort separately. FPS, free plasma protein S; PS, total plasma protein S, PROS1, protein S gene.
The complement binding protein gene cluster containing C4BPB and C4BPA mapped as new genetic risk factor for venous thrombosis (VT) by means of genome-wide association mapping studies (GWAS) in the GAIT cohort (1), a VT-risk group (2), targeted SNP typing in the GHS study (3), single SNP case-control analyses (rs3813948) of the MARTHA and FARIVE cohorts (4). Key linkage disequilibrium (LD) relationships between key SNPs employed by Buil et al in the context of studies. Significant support and key nonsignificance of assays are shown as red and blue, respectively. Significance was observed only for a combined analysis of both cohorts, but not for each cohort separately. FPS, free plasma protein S; PS, total plasma protein S, PROS1, protein S gene.
Protein S has been an ever-popular candidate locus for venous thrombosis because it confers high risk in human subjects with hereditary protein S deficiency.3 However, in persons lacking any of the well-characterized mutations, variations in protein S fail to explain much of the more subtle range of quantitative variations in susceptibility to venous thrombosis in the general population.
In contrast, C4BP previously had only been mentioned in the context of venous thrombosis by following the “guilty-by-association” principle.4 Generally, complement inactivator proteins, which include C4BP, are thought of as involved in immune response and inflammation.4 Interestingly, whereas the molecular bond between C4BP and protein S has led to the implication of the latter protein in immune response and inflammation, the reverse has not been a popular hypothesis; that is, that the binding of C4BP to protein S implicates C4BP in venous thrombosis. C4BP was mostly noted as a “piggyback” protein of protein S. However, once the inactivity of C4BP-bound protein S was established, a possible role for C4BP in the context of venous thrombosis emerged.5 The possibility that C4BP can be independently active in the coagulation pathway was noted in an earlier study as well.6 Now Buil et al are suggesting that, indeed, in the case of venous thrombosis, C4BP merits to be viewed as an active participant rather than a passive associate of protein S.
To be clear, the fact that protein S deficiency is a risk factor for venous thrombosis is not a matter of controversy.3 Based on my reading of the study, Buil et al are now proposing that the bonding between C4BP and protein S, compelling as it has been due to the function in blood coagulation, may have led to the impression that further investigations into any additional genetic factors with direct ties to this specific pathway were unnecessary.
In the broadest sense, genome-wide association studies liberate basic researchers from preexisting hypotheses so that even if particular loci have dominated discussions in the context of a disease, the genome-wide association approach provides an unbiased method to interrogate the genome for other significant loci. This approach has paid off; their results suggest that C4BP merits its own discussion, apart from protein S binding, as another avenue of investigation into the risk for venous thrombosis.1
While the C4BP locus emerged as an independent player on the stage of venous thrombosis from this study, its prominent molecular liaison, protein S, has dropped out of the picture, which feels a bit uncomfortable given the appreciation of the importance of this bond between the 2 proteins.4 Not only was protein S not present in the genome-wide association studies presented in the paper, but the authors went to some length to confirm that the SNPs were not associated with protein S levels. However, because each of the 4 study components was rather independent, with its own strengths and limitations, the evidence against protein S perhaps could have been stronger. Moreover, the authors rely heavily on the interpretation of linkage disequilibrium to connect study components that relied on different sets of SNPs, but in the end, leave the entire CBPB locus standing as a candidate (see figure). Linkage disequilibrium within and between the genes at the CBPB locus likely will render further fine-scale mapping attempts tricky.2 Finally, attempts to replicate or expand on this study need to consider that linkage disequilibrium between key SNPs might vary between study cohorts drawn from general populations of various genomic backgrounds.7
The work by Buil et al should set the stage for basic research into the unknown pathways that contribute to the risk of venous thrombosis in the general population. It is common for the variances in complex phenotypic trait values that can be explained by candidate SNPs to be underwhelming.2 Thus, the ability to account for approximately 11% of the variance in expression levels of C4BPA and plasma levels of unbound α7β0 should be looked upon as great success that is consistent with the authors' view that the study of the unbound C4BP α7β0 isoform could be a productive field for investigation on its own. However, although compelling, results by others indicate that the study of the interaction between protein S and C4BP continues to yield results of interest with regard to quantitative variation in protein S,8 and thus, that it may be premature to throw out the baby with the bath water.
In all, the result that the up-regulation of unbound C4b-complementary binding protein (C4BP), but not protein S, emerged from these analyses as a genetic risk factor for venous thrombosis is an unexpected but needed impetus for an expanded view of the factors involved in venous thrombosis. It also merits a shift from protein S–centric research. Despite the potential fallacies of array-based genome-wide association studies,2 in this and a few other cases a bird's-eye view of the genome has enabled Buil et al to spot a new target well worth pursuing on the ground.1,9,10
Conflict-of-interest disclosure: The author declares no competing financial interests. ■