Abstract
Dasatinib is an oral potent adenosine triphosphate (ATP)–competitive inhibitor of BCR-ABL, cKIT, platelet-derived growth factor receptor, and SRC family kinases (SFKs), which has demonstrated high efficiency in patients with imatinib-resistant chronic myelogenous leukemia. Here, we show that dasatinib weakly affects platelet activation by thrombin or adenosine diphosphate but is a potent inhibitor of platelet signaling and functions initiated by collagen or FcγRIIA cross-linking, which require immunoreceptor tyrosine-based activation motif phosphorylation by SFKs. Accordingly, dasatinib treatment rapidly decreases the volume of thrombi formed under arterial flow conditions in whole blood from patients or mice perfused over a matrix of collagen. Moreover, treatment of mice with dasatinib increases the tail bleeding time in a dose-dependent manner. Interestingly, these effects are rapidly reversible after interruption of the treatment. Our data clearly demonstrate that, in contrast to imatinib, dasatinib affects platelet functions in vitro and in vivo, which has important implications in clinic and could explain increased risks of bleeding observed in patients. Moreover, dasatinib efficiently prevents platelet activation mediated by FcγRIIA cross-linking and by sera from patients with heparin-induced thrombocytopenia, suggesting that reversible antiplatelet agents acting as ATP-competitive inhibitors of SFKs may be of therapeutic interest in the treatment of this pathology.
Introduction
Dasatinib is an oral potent adenosine triphosphate (ATP)–competitive inhibitor of tyrosine kinases, which has demonstrated high efficiency in patients with imatinib-resistant chronic myelogenous leukemia (CML).1–4 BCR-ABL is an oncogenic tyrosine kinase with deregulated activity linked to malignant transformation in CML, which has been first targeted with success in clinic by imatinib.5,6 Dasatinib is a second-generation tyrosine kinase inhibitor, more potent than imatinib on BCR-ABL and efficient on most of the imatinib-resistant ABL mutants.4 It is also effective on cKIT, platelet-derived growth factor (PDGF)–receptor and, in contrast to imatinib, on SRC family kinases (SFKs). Dasatinib is presently approved for all phases of Philadelphia-positive CML with resistance or intolerance to prior therapy, including imatinib. This compound is efficient and safe, although side effects, such as myelosuppression, gastrointestinal symptoms, rash, fluid retention syndromes, and bleeding episodes, are commonly observed.7 To cope with these side effects, most patients require reduction of the dose administrated or interruption of the treatment. Although the increased risk of bleeding under dasatinib therapy is clearly established with reported fatal brain hemorrhages, subdural hematomas, and gastrointestinal bleeding, it is difficult to ascertain to which degree the drug or the disease is responsible for the high incidence of bleeding. Moreover, the effect of dasatinib on platelet functions remains poorly characterized.
Blood platelets are the first line of defense against hemorrhages. On disruption of the vascular integrity, they adhere to exposed subendothelial matrix proteins and aggregate to stop bleeding. Platelet activation via soluble or immobilized agonists is a highly regulated process that involves several signaling proteins, including nonreceptor tyrosine kinases, such as Syk, BTK, FAK, and the SFKs. Six members of the SFK have been shown to be expressed in platelets, including c-Src, Lyn, Fgr, Fyn, Lck, and Yes.8 Besides its effect on platelet production and/or turnover leading, in some cases, to thrombocytopenia,7 dasatinib may also affect platelet functions through inhibition of SFKs. Consistent with this hypothesis, gastrointestinal hemorrhages have been reported in patients with normal platelet counts.
Under arterial shear rate, interaction of platelets with the disrupted vessel wall is largely mediated by binding of subendothelial von Willebrand factor (VWF) to its membrane receptor on platelets, the glycoprotein Ib-IX (GPIb-IX). Recent data indicate that the SFK Lyn mediates VWF/GPIb-IX-V–induced platelet activation.9 Moreover, maximal platelet responses to vascular injury require the contribution of integrins such as α2β1 or αIIbβ3 and of platelet membrane receptors bearing immunoreceptor tyrosine-based activation motif (ITAM), including glycoprotein VI-Fc receptor γ-chain (FcRγ) and FcγRIIA.10 These receptors and integrins activate cell signaling via nonreceptor tyrosine kinases. It is now clearly established that SFKs play a critical role in platelet activation downstream of the collagen receptor glycoprotein VI11,12 and FcγRIIA13,14 and contribute to GPIb9,15 and αIIbβ3-dependent signaling.16 On collagen/glycoprotein VI interaction, SFKs induce phosphorylation of conserved tyrosine residues within the ITAM of the FcRγ-chain providing a docking site for the tyrosine kinase Syk. The specific interaction of Syk with the ITAM leads to its stimulation, an early key step of platelet activation via glycoprotein VI or FcγRIIA.11,17 During αIIbβ3 integrin outside-in signaling, SFKs are also activated and have been proposed to mediate tyrosine phosphorylation of 2 residues of the β3 chain10,18 in addition to phosphorylate other signaling molecules. Consistent with these observations, SFKs have been shown to play an important role in supporting fibrin clot retraction,19 a mechanism requiring efficient αIIbβ3-mediated outside-in signaling.20
In this study, we analyzed the effect of the SFKs inhibitor dasatinib on platelet functions in vitro, ex vivo, and in vivo, both in mice and in humans. We demonstrate that, in contrast to imatinib, dasatinib impairs collagen-induced signaling and platelet aggregation, and induces hemostasis defects in mice. The formation of thrombi under physiologic arterial flow conditions ex vivo is also affected when patients are treated with dasatinib. Interestingly, these effects are rapidly reversible after interruption of the treatment. Moreover, we provide evidence that dasatinib efficiently prevents FcγRIIA-mediated platelet activation, suggesting that this compound or related ones could be of therapeutic interest in the treatment of heparin-induced thrombocytopenia (HIT). Altogether, these data have potential implications in clinic and may serve as a basis for the development of reversible antiplatelet agents acting as ATP-competitive inhibitors of SFKs.
Methods
Materials
The mouse monoclonal antiphosphotyrosine (clone 4G10) was from Upstate Biotechnology; the anti-phospho-Src (Y416) and the peroxidase-conjugated secondary antibodies were from Cell Signaling Technology. The anti-FcγRIIA monoclonal antibody IV.3 was from Medarex and the anti–mouse IgG F(ab′)2 to cross-link FcγRIIA/ IV.3 complexes was from Jackson ImmunoResearch Laboratories. All other antibodies and reagents were purchased from Sigma-Aldrich unless otherwise indicated. Dasatinib (Sprycel; Bristol-Myers Squibb) was kindly provided by Bristol-Myers Squibb.
Human blood samples.
Samples were obtained from healthy donors or patients diagnosed at the Department of Hematology, Toulouse University Medical Center (Toulouse, France), after informed consent was obtained in accordance with the Declaration of Helsinki. The Institutional Review Board of the Hôpital Purpan (Toulouse, France) approved the study. The characteristics of patients treated with dasatinib are provided in Table 1. The platelet counts of healthy donors were between 156 g/L and 310 g/L.
HIT serum samples.
HIT was identified in patients according to standard criteria,21 including the presence of antiplatelet factor 4/heparin antibodies and a positive platelet aggregation test in the presence of heparin. Sera were stored at −80°C until use. Before processing, they were heated at 56°C for 1 hour and centrifuged to remove any residual thrombin activity.
Preparation and activation of human platelets.
For in vitro aggregation experiments, blood was collected into acid-citrate-dextrose, and platelets were isolated by successive centrifugation steps essentially as described previously.22 Briefly, they were washed in a washing buffer (pH 6.5) containing 140 mmol/L NaCl, 5 mmol/L KCl, 5 mmol/L KH2PO4, 1 mmol/L MgSO4, 10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5 mmol/L glucose, and 0.35% bovine serum albumin (wt/vol). The same buffer containing 1 mmol/L CaCl2 was added to the final suspension, and pH was adjusted to 7.4. Before stimulation, platelets were preincubated (or not) for 15 minutes with dasatinib. For adenosine diphosphate (ADP) stimulation, platelets were activated in platelet-rich plasma.
Cross-linking of the low-affinity receptor for IgG, FcγRIIA, was performed by preincubation of platelets for 1 minute with the monoclonal antibody IV.3 (2 μg/mL) followed by addition of anti–mouse IgG F(ab′)2 (30 μg/mL) at 37°C under gentle shaking as described previously.23 For activation of platelets by HIT serum, 180 μL of platelet suspension was incubated with heparin (0.5 IU) and HIT sera (90 μL).
Platelet aggregation experiments were monitored by a turbidimetric method using a dual-channel aggregometer with continuous stirring at 900g at 37°C. P-selectin expression was measured as previously described,24 and ATP secretion was recorded by measuring the luminescence from the firefly luciferin-luciferase reaction using the Chrono-log aggregometer (Kordia).
For the analysis of the whole platelet tyrosine phosphorylation pattern, platelet stimulations were stopped by addition of ristocetin-induced platelet aggregation buffer, protein submitted to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred onto nitrocellulose, and the tyrosine phosphorylated proteins were detected by immunoblotting with the 4G10 antiphosphotyrosine antibody.
Animals.
Wild-type mice were of C57Bl/6 genetic background. They were housed in the IFR-Toulouse Purpan animal facility according to institutional guidelines. For all experiments, 5- to 10-week-old mice were used. Ethical approval for animal experiments was received from the French Ministry of Research in agreement with the European Union guidelines. Oral administration of dasatinib ranged from 1.25 mg/kg to 5 mg/kg as indicated.
In vitro model of thrombosis on immobilized collagen under flow conditions.
Glass microcapillaries were coated with 500 μg/mL type I collagen from equine tendon for 1 hour at 37°C. The flow chamber, mounted on an epifluorescence microscope (Axiovert 200; Carl Zeiss), allowed direct visualization of platelet adhesion and aggregation processes, which were recorded with a CCD camera (Cool Snap HQ, Roper Scientific). Human or mouse blood was drawn into lepirudine (200 IU/mL), and DiOC6 (2 μM, 30 minutes at 37°C) was used to label platelets in whole blood. Labeled blood was then perfused through collagen-coated glass microcapillaries for 2 minutes at a wall shear rate of 1500 seconds (15 dyne/cm2), followed by washing for 2 minutes at the same shear rate with phosphate-buffered saline. Thrombus formation was visualized with a 40× long working distance objective in real time (acquisition rate, 1 frame every 5 seconds) for both fluorescent and transmitted light microscopy as described.24 Image sequences of the time lapse recording and analysis of surface coverage were performed using the Metamorph software (Universal Imaging Corp). After deconvolution, a lower intensity threshold was applied to distinguish platelets from the background, and the similar threshold was then used to analyze all Z-stack acquired for a given experiment. Thrombus volume was calculated as the summation of partial volumes measured from the area occupied by platelets in each plane of Z-stacks.
Lipid extraction and analysis.
Platelets were labeled with 0.6 mCi/mL [32P]orthophosphate during 45 minutes in a phosphate-free N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-Tyrode buffer (pH 6.5) at 37°C. 32P-Labeled platelets were then washed once in the same buffer and suspended at a final concentration of 109 platelets/mL in modified N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-Tyrode buffer (pH 7.38). After stimulation, reactions were stopped by addition of chloroform/methanol (vol/vol) containing 0.6 N HCl, and lipids were immediately extracted and analyzed by a combination of thin-layer chromatography and high-performance liquid chromatography as described previously.23
Tail bleeding time
We measured bleeding time by 3-mm tail-tip transection in mice anesthetized by an intraperitoneal injection of a mixture of ketamine (25 mg/kg) and xylazine (10 mg/kg). A stopwatch was started immediately on transection to determine the time required for the bleeding to stop. Blood drops were removed every 15 seconds with the use of a paper filter. If bleeding did not resume within 30 seconds of cessation, it was considered stopped.
Results
Dasatinib inhibits collagen-induced platelet activation
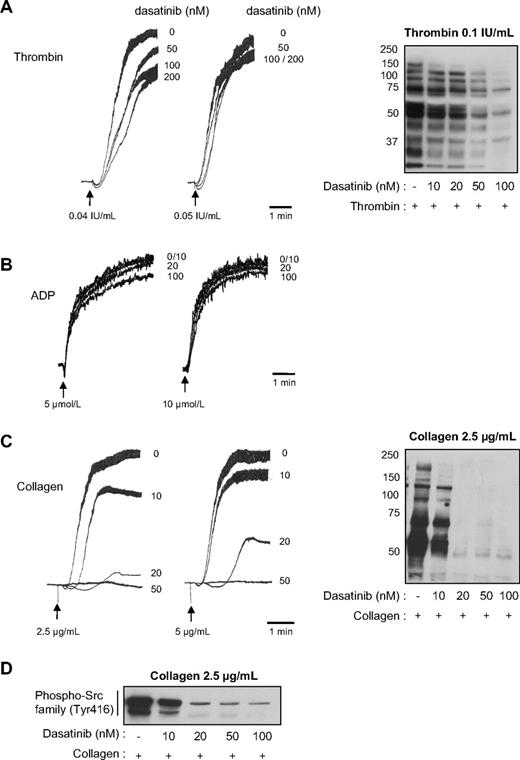
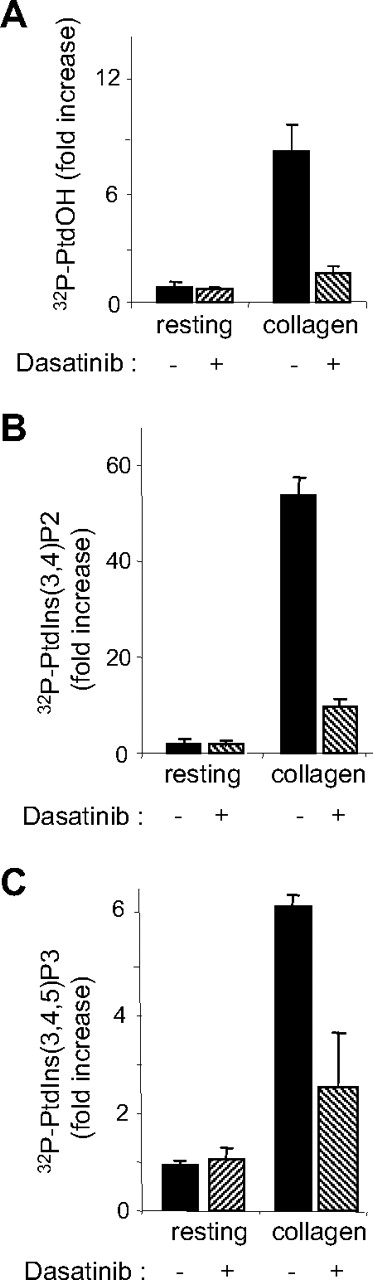
To address the effect of dasatinib on human platelet responses, washed platelets were treated with increasing doses of dasatinib and stimulated by different concentrations of thrombin, ADP, or collagen. Dasatinib significantly inhibited platelet aggregation in response to low concentrations of thrombin but only weakly affected aggregation at higher concentrations of agonist (Figure 1A). P-selectin expression and ATP secretion induced by thrombin were not significantly reduced (12.2% ± 2% and 0.5% ± 10% inhibition at 50 nM dasatinib in response to 0.1 IU/mL thrombin, respectively). Dasatinib, even at 200 nM, had hardly any detectable effect on platelet aggregation induced by 0.1 IU/mL thrombin (not shown), whereas the pattern of tyrosine phosphorylation was strongly affected in a dose-dependent manner by the drug (Figure 1A right panel). These data suggest that dasatinib affects αIIbβ3 outside-in signaling, which is known to involve SFKs10,16 and accounts for a major part of tyrosine phosphorylation in response to thrombin in aggregating conditions. ADP-induced platelet aggregation in platelet-rich plasma was not significantly affected by dasatinib (Figure 1B). Interestingly, dasatinib treatment very efficiently inhibited platelet aggregation in response to collagen. Indeed, 50 nM of the drug abolished platelet shapes change and aggregation in response to all collagen concentrations tested (Figure 1C). P-selectin expression and ATP secretion were also clearly decreased (86.5% ± 2% and 60% ± 5% inhibition at 50 nM dasatinib in response to 5 μg/mL collagen, respectively). Under these conditions, tyrosine phosphorylations were strongly inhibited (Figure 1C right panel). Moreover, using a phosphospecific antibody recognizing the active form of SFKs, we show that dasatinib efficiently inhibited its targets (Figure 1D). To further investigate the effect of dasatinib on signaling events initiated by collagen, we analyzed 2 critical pathways downstream of glycoprotein VI: the phospholipase Cγ (PLCγ) and the phosphoinositide 3-kinase (PI 3-kinase) pathways. Phosphatidic acid (PtdOH) production, which reflects the activity of PLC and diacylgycerol kinase (DAG-kinase) in platelets, was strongly inhibited by 50 nM dasatinib (Figure 2A). Conversely, thrombin-induced PtdOH synthesis, which is known to use the Gq/PLCβ/DAG-kinase pathway, was not affected (105% ± 10% of control, n = 3). The production of D3-phosphoinositides by PI 3-kinase was also very efficiently inhibited in response to collagen (Figure 2B-C). Altogether, these results indicate that dasatinib has a relatively modest impact on thrombin or ADP-induced washed platelet aggregation, whereas collagen-induced signaling and platelet responses are strongly inhibited by clinically relevant doses of the drug. Indeed, after 2 to 5 hours of treatment, the concentration of dasatinib can reach approximately 150 nM and 100 nM in plasma of patients receiving the 140-mg or the 70-mg regimen, respectively.25 Interestingly, imatinib acting on PDGF-R, cKit, and ABL, but not on SFKs, had no detectable effects on washed platelet aggregation in response to thrombin or collagen (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Even at high concentrations, imatinib did not affect the tyrosine phosphorylation pattern of collagen-activated platelets (supplemental Figure 1), whereas at similar doses it blocked tyrosine phosphorylation in K562 cells expressing BCR-ABL (not shown).
Effect of dasatinib on human platelet aggregation and tyrosine phosphorylation on thrombin or collagen stimulation. Platelets from healthy donors were treated or not with increasing concentrations of dasatinib for 15 minutes and stimulated with (A) thrombin, (B) ADP, or (C) collagen. Platelet aggregation was assessed using a Chrono-log dual-channel aggregometer under stirring at 900g. The aggregation profiles shown are representative of 3 independent experiments. Right panels show the effect of dasatinib on whole platelet tyrosine phosphorylation pattern in response to thrombin or collagen. Results are representative of 3 independent experiments. (D) Western blot analysis of phospho-Src family (Tyr416) in human platelets incubated with increasing doses of dasatinib and stimulated by 2.5 μg/mL collagen for 3 minutes. The Western blots shown are representative of 3 independent experiments.
Effect of dasatinib on human platelet aggregation and tyrosine phosphorylation on thrombin or collagen stimulation. Platelets from healthy donors were treated or not with increasing concentrations of dasatinib for 15 minutes and stimulated with (A) thrombin, (B) ADP, or (C) collagen. Platelet aggregation was assessed using a Chrono-log dual-channel aggregometer under stirring at 900g. The aggregation profiles shown are representative of 3 independent experiments. Right panels show the effect of dasatinib on whole platelet tyrosine phosphorylation pattern in response to thrombin or collagen. Results are representative of 3 independent experiments. (D) Western blot analysis of phospho-Src family (Tyr416) in human platelets incubated with increasing doses of dasatinib and stimulated by 2.5 μg/mL collagen for 3 minutes. The Western blots shown are representative of 3 independent experiments.
Effect of dasatinib on collagen-induced PLC/DAG-kinase and PI 3-kinase activation. 32P-labeled platelets were treated or not with 50 nM dasatinib for 15 minutes and stimulated by 5 μg/mL collagen for 2 minutes under stirring at 900g, and the levels of (A) 32P-PtdOH, (B) 32P-PtdIns(3,4)P2, and (C) 32P-PtdIns(3,4,5)P3 were analyzed as indicated in “Methods.” Results are mean ± SEM of 3 independent experiments.
Effect of dasatinib on collagen-induced PLC/DAG-kinase and PI 3-kinase activation. 32P-labeled platelets were treated or not with 50 nM dasatinib for 15 minutes and stimulated by 5 μg/mL collagen for 2 minutes under stirring at 900g, and the levels of (A) 32P-PtdOH, (B) 32P-PtdIns(3,4)P2, and (C) 32P-PtdIns(3,4,5)P3 were analyzed as indicated in “Methods.” Results are mean ± SEM of 3 independent experiments.
Dasatinib treatment affects thrombus formation under flow
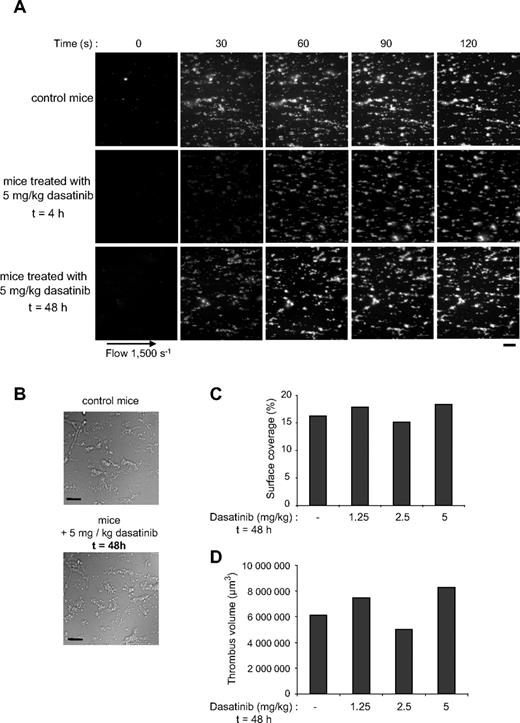
To further investigate the effect of dasatinib on human platelets, we compared thrombus formation under physiologic flow conditions in whole blood from healthy donors and from patients treated with dasatinib or imatinib. Normal blood was used as a control in these experiments because the patients tested were all under complete hematologic response (Table 1). Moreover, because imatinib had no significant effect on washed platelet activation (supplemental Figure 1), we compared the effect of dasatinib treatment of CML patients to that of imatinib. The drugs were administered 4 to 5 hours before blood collection. Whole blood containing fluorescent labeled platelets was perfused over a matrix of collagen at an arterial shear rate of 1500 seconds (15 dyne/cm2) during 2 minutes. Blood from the healthy donors reproducibly formed densely packed platelet thrombi on collagen fibers, whereas blood from dasatinib-treated patients formed much smaller thrombi (Figure 3; Table 1). Both the surface covered by platelet aggregates (not shown) and the thrombi volume were significantly reduced (Figure 3C). Patients treated with imatinib showed a reduction of thrombus growth compared with healthy donors, but the effect of dasatinib was much more pronounced (Figure 3C).
Rapid and dose-dependent effect of dasatinib treatment on platelet thrombus formation on collagen under arterial flow conditions in humans and mice. DiOC6-labeled platelets in whole blood from healthy donor or patients 1 to 4 treated with dasatinib 4 hours earlier were perfused through a collagen-coated microcapillary at a shear rate of 1500 seconds for 2 minutes. Thrombus formation was visualized with a 40× long working distance objective in real time and then imaged using transmitted light microscopy. Representative images are shown (A). After a washing step with phosphate-buffered saline for 2 minutes at the same shear rate to remove nonadherent cells, slides were visualized using differential interference contrast microscopy, and representative images are shown (B). Images were visualized using an epifluorescence microscope (Axiovert 200; Carl Zeiss Inc); 40×/1.3 NA oil objective, no solution, with DiOC6. Images were acquired using a charge-coupled device camera (Cool Snap HQ; Roper Scientific) and Metamorph Version 6.21.6 software. Thrombus volume (C) was measured at 2 surface locations in each of the 4 independent experiments performed from healthy donors, dasatinib-treated patients, and imatinib-treated patients (mean ± SEM). **Significant difference (P < .005), according to Student t test. Scale bar represents 20 μm. The same experiments were performed using whole blood from mice treated with 1.25, 2.5, or 5 mg/kg dasatinib (5 mice per group) 4 hours before blood collection, and the thrombus volumes were measured (D). Results shown are representative of 2 independent experiments.
Rapid and dose-dependent effect of dasatinib treatment on platelet thrombus formation on collagen under arterial flow conditions in humans and mice. DiOC6-labeled platelets in whole blood from healthy donor or patients 1 to 4 treated with dasatinib 4 hours earlier were perfused through a collagen-coated microcapillary at a shear rate of 1500 seconds for 2 minutes. Thrombus formation was visualized with a 40× long working distance objective in real time and then imaged using transmitted light microscopy. Representative images are shown (A). After a washing step with phosphate-buffered saline for 2 minutes at the same shear rate to remove nonadherent cells, slides were visualized using differential interference contrast microscopy, and representative images are shown (B). Images were visualized using an epifluorescence microscope (Axiovert 200; Carl Zeiss Inc); 40×/1.3 NA oil objective, no solution, with DiOC6. Images were acquired using a charge-coupled device camera (Cool Snap HQ; Roper Scientific) and Metamorph Version 6.21.6 software. Thrombus volume (C) was measured at 2 surface locations in each of the 4 independent experiments performed from healthy donors, dasatinib-treated patients, and imatinib-treated patients (mean ± SEM). **Significant difference (P < .005), according to Student t test. Scale bar represents 20 μm. The same experiments were performed using whole blood from mice treated with 1.25, 2.5, or 5 mg/kg dasatinib (5 mice per group) 4 hours before blood collection, and the thrombus volumes were measured (D). Results shown are representative of 2 independent experiments.
To check whether the effect of dasatinib was dose dependent, we treated mice with increasing doses (1.25-5 mg/kg) of dasatinib. Figure 3D shows that the thrombus volume obtained under physiologic shear rate conditions decreased progressively according to the dose of dasatinib ingested.
Overall, these results indicate that, under physiologic arterial shear rate, dasatinib treatment rapidly affects thrombus formation on collagen fibers both in mice and humans.
Dasatinib treatment reversibly increases mouse bleeding time
To investigate the effect of dasatinib on primary hemostasis in vivo, we analyzed the tail bleeding time of mice treated by increasing concentrations of the drug. Four hours after ingestion of dasatinib, bleeding induced by amputation of the tail tip increased in a dose-dependent manner (Figure 4A). Twenty-four hours after ingestion, the effect of dasatinib decreased, especially when low doses were administered (Figure 4B). Interestingly, even at high doses, the effect of dasatinib was no longer detectable 48 hours after ingestion (Figure 4C). These results indicate a rapid reversibility of the effect of dasatinib on primary hemostasis in vivo and are consistent with the pharmacokinetics of the drug described in mice.25 Accordingly, the thrombus surface and volume obtained under physiologic shear rate conditions in whole blood was comparable in control mice and in mice treated with a single dose of dasatinib 48 hours before measurement (Figures 3E, 5). Moreover, we could compare ex vivo the thrombus formation in 2 patients at 4 and 24 hours after ingestion of dasatinib (supplemental Figure 2). Again, the reversibility of dasatinib action was clearly observed. After 24 hours, the thrombi formed on collagen fibers were much bigger in surface and volume than the thrombi formed 4 hours after treatment. The pharmacokinetics of dasatinib in humans appears consistent with the rapid reversibility of the effect. Indeed, the plasma concentration peaks 2 to 5 hours after drug intake and then decreases progressively to become undetectable after 24 hours.25 Altogether, these data show clearly that dasatinib rapidly and reversibly affects platelet functions and hemostasis.
Reversible effect of dasatinib over the time on tail bleeding time of mice. The tail bleeding time of control mice and mice treated with dasatinib 4 (A), 24 (B), and 48 hours (C) after ingestion of the drug. The single oral administration of dasatinib was 1.25, 2.5, or 5 mg/kg, as indicated.
Reversible effect of dasatinib over the time on tail bleeding time of mice. The tail bleeding time of control mice and mice treated with dasatinib 4 (A), 24 (B), and 48 hours (C) after ingestion of the drug. The single oral administration of dasatinib was 1.25, 2.5, or 5 mg/kg, as indicated.
Reversible effect of dasatinib on platelet thrombus formation under arterial flow conditions. DiOC6-labeled platelets in whole blood from control mice or mice treated with dasatinib (5 mg/kg) 4 or 48 hours before blood collection were perfused through a collagen-coated microcapillary at a shear rate of 1500 seconds for 2 minutes. Thrombus formation was visualized and analyzed as in Figure 3. Representative images of platelet adhesion from control and treated mice are shown (A-B). Scale bar represents 20 μm. Area covered by platelet thrombi (C) and thrombus volume (D) were measured at 2 surface locations in each of 3 different conditions 48 hours after oral administration of dasatinib (1.25, 2.5, or 5 mg/kg as indicated). Results shown are mean values of 2 independent experiments.
Reversible effect of dasatinib on platelet thrombus formation under arterial flow conditions. DiOC6-labeled platelets in whole blood from control mice or mice treated with dasatinib (5 mg/kg) 4 or 48 hours before blood collection were perfused through a collagen-coated microcapillary at a shear rate of 1500 seconds for 2 minutes. Thrombus formation was visualized and analyzed as in Figure 3. Representative images of platelet adhesion from control and treated mice are shown (A-B). Scale bar represents 20 μm. Area covered by platelet thrombi (C) and thrombus volume (D) were measured at 2 surface locations in each of 3 different conditions 48 hours after oral administration of dasatinib (1.25, 2.5, or 5 mg/kg as indicated). Results shown are mean values of 2 independent experiments.
Dasatinib prevents platelet activation by serum from HIT patients
To test whether dasatinib may have a potential therapeutic interest in antiplatelet therapy, we analyzed its effects on platelet activation via clustering of FcγRIIA by a specific antibody (IV.3) and a secondary IgG F(ab′)2 fragment or by serum from HIT patients. FcγRIIA is a member of the immunoglobulin gene superfamily and is composed of 2 extracellular Ig homology domains and a cytoplasmic tail bearing an ITAM, which becomes tyrosine phosphorylated by SFKs on receptor clustering. The tyrosine phosphorylation of FcγRIIA by SFKs is a critical step in FcγRIIA-dependent platelet activation.10 The importance of FcγRIIA in pathophysiologic disorders such as HIT is well established,26 but it may also play a role in physiologic platelet activation processes.27,28
FcγRIIA cross-linking by the specific antibody IV.3 induced an efficient aggregation of washed platelets that was fully inhibited by 50 nM dasatinib (Figure 6A). Interestingly, dasatinib also inhibited platelet aggregation induced by sera from HIT patients in a dose-dependent manner (Figure 6B). At low doses of dasatinib (< 50 nM), platelet aggregation was strongly delayed; whereas at higher concentrations, platelet aggregation was fully abolished. These results are consistent with the well-described role of SFKs in FcγRIIA-dependent platelet activation and show that dasatinib can block this process at clinically achievable doses.
Dasatinib efficiently blocks platelet aggregation induced by direct FcγRIIa clustering or by sera from HIT patients. Platelets from healthy donors were treated or not with increasing concentrations of dasatinib and stimulated by FcγRIIA clustering by incubation of platelets with (A) 2 μg/mL of monoclonal antibody IV.3 for 1 minute followed by addition of 30 μg/mL goat anti–mouse F(ab′)2 or with (B) HIT sera and heparin (0.5 IU). Platelet aggregation was assessed using a Chrono-log dual-channel aggregometer under stirring at 900g. The aggregation profiles shown are representative of 3 or 4 independent experiments.
Dasatinib efficiently blocks platelet aggregation induced by direct FcγRIIa clustering or by sera from HIT patients. Platelets from healthy donors were treated or not with increasing concentrations of dasatinib and stimulated by FcγRIIA clustering by incubation of platelets with (A) 2 μg/mL of monoclonal antibody IV.3 for 1 minute followed by addition of 30 μg/mL goat anti–mouse F(ab′)2 or with (B) HIT sera and heparin (0.5 IU). Platelet aggregation was assessed using a Chrono-log dual-channel aggregometer under stirring at 900g. The aggregation profiles shown are representative of 3 or 4 independent experiments.
Discussion
Blood platelets are critical to maintain normal hemostasis and key players in atherothrombosis. On vascular injury, subendothelial matrix proteins, such as collagen, are exposed and interact with VWF, allowing platelet arrest and subsequent spreading, activation, and secretion of soluble mediators. These soluble agonists recruit circulating platelets, allowing aggregation and thrombus growth for the bleeding to stop. Current antiplatelet drugs interfere with important steps in their activation process, including thromboxane A2 synthesis, P2Y12 ADP receptor activation, or αIIbβ3 integrin engagement.29,30 So far, intracellular signaling has not been targeted by antiplatelet drugs in clinic. Platelet activation is a highly dynamic and regulated process involving many membrane receptors and several intracellular signaling cascades. Protein tyrosine kinases are highly expressed in platelets and play a critical role in their activation process.31 Many of the key platelet membrane receptors recruit and activate nonreceptor tyrosine kinases, particularly SFKs, leading to robust tyrosine phosphorylation of several proteins.32 Some studies have reported the blocking effects of tyrosine kinase inhibitors on different platelet functions.33–35 However, most of the tyrosine kinase inhibitors used so far were nonselective, and it is still difficult to ascertain a role for the different tyrosine kinases present in platelet. Interestingly, the increasing development of small-molecule inhibitors of kinases, particularly in targeted cancer therapy, allow us now to test more selective compounds. Imatinib targeting ABL, cKit, and PDGF-R and more recently dasatinib targeting ABL, cKit, PDGF-R, and also SFKs have strongly improved the treatment of CML.1 Strikingly, among the adverse effects of dasatinib, bleeding of different types, including epistaxis, gastrointestinal and brain hemorrhages have been observed.7 Here, we demonstrate that, besides its thrombocytopenic effect reported in some cases, which could be explained by the contribution of tyrosine-kinases in megakaryocytopoiesis,36 dasatinib also strongly affects platelet functions in vitro and in vivo. This might explain the gastrointestinal hemorrhages observed in patients with normal platelet counts. Our data clearly show that, in contrast to imatinib, which has no detectable effects on washed platelet responses induced by collagen or thrombin, dasatinib strongly inhibits collagen-induced platelet activation and affects platelet aggregation in response to low doses of thrombin. The striking difference between the effect of imatinib and dasatinib on platelet activation in vitro suggests that the major effect of dasatinib is mediated by SFK inhibition, although other potential targets of the drug, such as ephrin receptor, may also contribute. Dasatinib is highly efficient in blocking signal transduction mechanisms in response to collagen, including tyrosine phosphorylation, PI 3-kinase activation, and PLC/DAG-kinase activation. This is consistent with the critical role of SFKs, mainly Fyn and Lyn, in the phosphorylation of FcRγ-chain, an early mandatory step in the signaling cascade initiated by glycoprotein VI triggering.18,37 Fyn and Lyn constitutively interact with the proline-rich domain of the glycoprotein VI cytosolic tail; and on cross-linking, these kinases become active and phosphorylate the FcRγ-chain ITAM. This initial step allows Syk recruitment and activation and the subsequent phosphorylation of several downstream targets, including LAT and SLP76, 2 adaptor molecules essential for PLCγ2 and PI 3-kinase activation.18,38 Dasatinib also affects thrombus formation measured ex vivo by microscopy real-time imaging. Whole blood samples from patients treated with dasatinib were perfused under arterial flow over collagen fibers. In these conditions, the thrombotic process involves the tethering of platelets via GPIb-IX-V to VWF bound to collagen and the interaction of glycoprotein VI and α2β1 with collagen to mediate stable adhesion.39 Platelet thrombus growth and stabilization are then linked to secretion and aggregation via αIIbβ3. SFKs are important in different phases of this highly dynamic and coordinated process.9,27,40 This is consistent with the fact that both the surface covered by the thrombi and their volume are affected by dasatinib. In washed platelets, high concentrations of dasatinib (100 nM) strongly inhibit tyrosine phosphorylation under aggregating conditions in response to high doses of thrombin, suggesting that tyrosine kinases activated via αIIbβ3 outside-in signaling are affected. This was not sufficient to reverse aggregation under stirring conditions but may contribute to the reduction of thrombus growth under arterial flow conditions.
In vivo, 4 hours after ingestion, dasatinib increases the tail bleeding time in a dose-dependent manner in mice, and this effect is rapidly reversible after interruption of the treatment. This reversible effect could also be measured ex vivo using blood samples from treated mice to follow the thrombus formation under arterial shear rate. Importantly, this reversibility of dasatinib effect could be measured in 2 patients 24 hours after dasatinib intake. A very recent clinical study also reports a reversible effect of dasatinib on platelet responses in CML patients.41 Altogether, these results demonstrate that dasatinib is affecting platelet functions in vitro, ex vivo, and in vivo, which has potential implications in clinic. It is particularly interesting to consider the rapid and reversible effect of dasatinib on platelet functions in vivo, suggesting that invasive surgical gesture should be avoided during the first hours after dasatinib intake.
One important question is the relevance of tyrosine kinase inhibitors as antiplatelet drugs. Previous studies33–35 have addressed this question using nonselective tyrosine kinase inhibitors; however, it is difficult to draw clear conclusions. Very recent data suggest that selective Syk inhibitors may be of interest as antiplatelet agents to prevent thrombosis42 or HIT.43 Here we show that SFK inhibition by dasatinib impairs FcγRIIA-mediated platelet activation. HIT is a drug-induced immune thrombocytopenia where antibodies reactive with heparin-PF4 complexes lead to FcγRIIA-mediated platelet activation. Dasatinib efficiently prevents platelet activation induced by HIT antibody from patients at clinically achievable doses. These results strongly suggest that dasatinib or related compounds acting as SFK inhibitors could be of interest as active agents in HIT treatment. The rapid efficiency of dasatinib and its reversibility of effect could be important advantages to achieve optimal treatment of HIT. The use of the transgenic mouse model expressing the human FcγRIIA would allow to test dasatinib in vivo to further validate this drug or related once on an animal model of HIT.44 Moreover, defining which SFK member is involved in FcγRIIA ITAM phosphorylation and the degree of redundancy of these kinases to undergo this key event upstream of Syk activation would be important to design selective inhibitors of this pathway. Whether dasatinib or related SFKs inhibitors could be of interest as potential new therapy for thrombosis or other vascular obstructive diseases remain to be established using appropriate animal models, but the increased risk of bleeding must be taken into consideration. In this respect, the development of inhibitors selectively targeting the different SFK members could be useful to elaborate therapeutic strategies to specifically modulate appropriate platelet functions.
In conclusion, using complementary approaches, we have demonstrated that inhibition of SFKs by dasatinib treatment rapidly and reversibly affects platelet activation by ITAM-mediated signaling and induces hemostatic defects. We also provide evidence that this drug efficiently prevents FcγRIIA-mediated platelet activation, suggesting that this compound or related ones acting as ATP-competitive inhibitors of SFKs could be of therapeutic interest in the treatment of HIT.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Plantavid, F. Gaits-Iacovoni, S. Manenti, H. Tronchère, and H. Mohamed for stimulating discussions and for critical reading of the manuscript.
This work was supported by Inserm, ANR (program Jeunes Chercheurs, Jeunes Chercheuses; no. ANR-07-JCJC-0093-01), INCa, and Région Midi-Pyrénées.
Authorship
Contribution: M.-P.G, V.M., and M.-C.V. designed and performed most experiments and analyzed data; S.A. performed microscopy; C.G. and P.S. selected and prepared HIT sera and performed the experiments on FcγRIIA; and C.R. and B.P. designed research, supervised the work, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernard Payrastre, Inserm, U563, Centre Hospitalier Universitaire Purpan, BP 3028, 31024 Toulouse Cedex 03, France; e-mail: bernard.payrastre@inserm.fr.
References
Author notes
*M.-P.G. and V.M. contributed equally to this work.