Abstract
Preclinical models have demonstrated the efficacy of granulocyte-macrophage colony-stimulating factor-secreting cancer immunotherapies (GVAX platform) accompanied by immunotherapy-primed lymphocytes after autologous stem cell transplantation in hematologic malignancies. We conducted a phase 2 study of this combination in adult patients with acute myeloid leukemia. Immunotherapy consisted of autologous leukemia cells admixed with granulocyte-macrophage colony-stimulating factor-secreting K562 cells. “Primed” lymphocytes were collected after a single pretransplantation dose of immunotherapy and reinfused with the stem cell graft. Fifty-four subjects were enrolled; 46 (85%) achieved a complete remission, and 28 (52%) received the pretransplantation immunotherapy. For all patients who achieved complete remission, the 3-year relapse-free survival (RFS) rate was 47.4% and overall survival was 57.4%. For the 28 immunotherapy-treated patients, the RFS and overall survival rates were 61.8% and 73.4%, respectively. Posttreatment induction of delayed-type hypersensitivity reactions to autologous leukemia cells was associated with longer 3-year RFS rate (100% vs 48%). Minimal residual disease was monitored by quantitative analysis of Wilms tumor-1 (WT1), a leukemia-associated gene. A decrease in WT1 transcripts in blood was noted in 69% of patients after the first immunotherapy dose and was also associated with longer 3-year RFS (61% vs 0%). In conclusion, immunotherapy in combination with primed lymphocytes and autologous stem cell transplantation shows encouraging signals of potential activity in acute myeloid leukemia (ClinicalTrials.gov: NCT00116467).
Introduction
Intensive chemotherapy regimens induce complete remission (CR) in the majority of adults less than age 60 with acute myeloid leukemia (AML), but maintenance of durable remissions remains a challenge. The optimal postremission treatment to eradicate residual leukemia is controversial. Treatment options include repeated cycles of intensive cytarabine-based consolidation chemotherapy,1 autologous stem cell transplantation (ASCT),2–4 and allogeneic stem cell transplantation (alloSCT).5 Mixed results with investigational immunotherapies (ie, interleukin-2,6 histamine dihydrochloride7 ) added to the aforementioned aggressive treatment approaches have been noted. Several large randomized studies have failed to demonstrate a clearly superior overall strategy for postremission treatment of AML, but alloSCT is often considered the treatment of choice for patients with high-risk disease and a suitable human leukocyte antigen–matched donor.4,5,8–10 ASCT offers the advantage over alloSCT of a lower transplantation-related mortality rate due to the absence of graft-versus-host disease but is associated with a higher relapse rate attributed to the absence of an immune-mediated graft-versus-leukemia effect. Strategies that combine the cytoreductive power of pre-ASCT myeloablative preparative regimens with immunotherapy to induce an antileukemia immune response could potentially result in durable remissions without the morbidity and mortality associated with allogeneic graft-versus-host disease.
GVAX refers to an immunotherapy platform in which whole tumor cells are modified to secrete granulocyte-macrophage colony-stimulating factor (GM-CSF). Preclinical studies have clearly demonstrated the induction of antitumor immunity and tumor regressions with the GVAX platform. Clinical trials exploring both autologous GVAX11–13 derived from individual patient tumors as well as allogeneic GVAX14–18 derived from established tumor cell lines have been conducted in multiple tumor types. In addition, several clinical studies have explored a hybrid approach of autologous tumor cells admixed with a GM-CSF–secreting allogeneic tumor cell line.19–22 This approach offers the potential advantage of including patient-specific tumor antigens with an “off-the-shelf” GM-CSF–secreting tumor cell immunotherapy product.
Preclinical studies exploring the use of a GM-CSF–secreting cellular immunotherapy in combination with myeloablative stem cell transplantation and adoptive transfer of immunotherapy “primed” lymphocytes have demonstrated enhanced antitumor immune and clinical activity with this approach compared with immunotherapy administered in the nontransplantation setting.23 This phase 2 trial was undertaken to explore the safety, immune, and clinical activity of immunotherapy based on the “mixed” GVAX platform (autologous leukemia cells mixed with GM-CSF–secreting K562 cells CG9962 or K562/GM) combined with ASCT and the adoptive transfer of immunotherapy-primed lymphocytes as postremission therapy for AML. In addition to immune response and clinical endpoints, analysis of minimal residual disease in blood and bone marrow was conducted throughout the study by using quantitative analysis of Wilms tumor-1 (WT1) gene expression. WT1 is a pan-leukemia marker overexpressed in the majority of acute and chronic leukemias. At the time of complete hematologic remission, despite morphologic clearance of leukemia cells from the blood and bone marrow, WT1 transcript levels remain detectable in many patients, and its persistence in the blood portends a poor prognosis.24–26 This analysis was included to monitor the immunotherapy-associated reductions in residual leukemia as a potential signal of antitumor activity in study patients without hematologic evidence of leukemia at the time of immunotherapy administration.
Methods
Study design
This was a phase 2, open-label, single-arm, multicenter study evaluating the addition of immunotherapy to an AML treatment program, including induction and consolidation chemotherapy followed by ASCT. The prespecified objectives were to assess safety, feasibility, GM-CSF pharmacokinetics, immune response, WT1 response, relapse-free survival (RFS), and overall survival (OS). The trial was approved by all participating institutional review boards, and all enrolled subjects gave written informed consent in accordance with the Declaration of Helsinki. The trial is registered at ClinicalTrials.gov (NCT00116467).
Patient population
Patients were beetween 18 and 60 years of age with de novo AML (> 20% marrow blasts and no preexisting hematologic disorder longer than 3 months), no prior leukemia therapy (except leukapheresis or < 72 hours of hydroxyurea), and creatinine less than 2.0 mg/dL. Patients were excluded for acute promyelocytic leukemia, extreme obesity (weight > 200% ideal body weight), severe heart disease precluding anthracyclines, other malignancies within 5 years, active autoimmune disease, and pregnancy. Patients who achieved CR were eligible to continue on the study and receive consolidation chemotherapy, immunotherapy, and ASCT. Eligibility to proceed to pretransplantation immunotherapy and ASCT included completion of protocol-directed consolidation chemotherapy, maintenance of CR, adequate autologous blood stem cell collection (> 2 × 106 CD34+ cells/kg), acceptable organ function, resolution of grade 3 or 4 adverse events, and no systemic corticosteroids within 14 days. Posttransplantation immunotherapy treatments were initiated at least 6 weeks after transplantation and required an absolute neutrophil count (ANC) greater than 1000/mm3, platelet count greater than 50 000/mm3, and hemoglobin level greater than 8 g/dL.
Study treatment
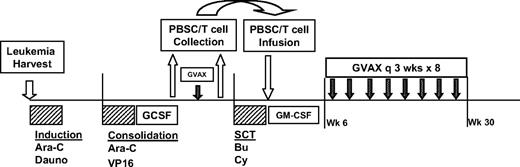
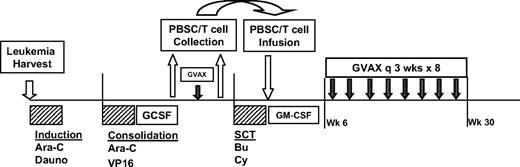
The study design is shown in Figure 1. All enrolled patients underwent an initial harvest of autologous leukemia cells from blood or bone marrow. These cells were then irradiated and admixed with the K562/GM cells to create the final immunotherapy product. Thereafter, patients received induction chemotherapy with high-dose cytarabine (2 g/m2 twice a day × 12 doses) and daunorubicin (60 mg/m2 every day × 3 doses). A second cycle of alternative investigator-selected induction chemotherapy was allowed if CR was not achieved. For patients ultimately achieving CR, one cycle of consolidation chemotherapy with cytarabine (2 g/m2 twice a day × 8 doses) and etoposide (40 mg/kg by continuous infusion over 96 hours concurrent with cytarabine) was administered, followed by collection of granulocyte-colony stimulating factor (GCSF)-mobilized (5-10 μg/kg daily beginning on day 14) peripheral blood stem cells (target >2 × 106 CD34+ cells/kg) by leukapheresis upon hematologic recovery. Approximately 2 weeks after stem cell collection, patients received a single pretransplantation immunotherapy treatment, followed 2 weeks later by a second leukapheresis to collect “primed” lymphocytes (target 108 CD3+ cells/kg). The ASCT was performed using a myeloablative preparative regimen of busulfan (0.8 mg/kg intravenously every 6 hours × 16 doses) and cyclophosphamide (60 mg/kg every day × 2 doses). Peripheral blood stem cells and “primed” lymphocytes were reinfused on transplantation day 0, followed by treatment with daily GM-CSF (125-250 μg) until neutrophil recovery (ANC >1500/mm3 × 3). Beginning at 6 weeks after transplantation (or upon adequate hematologic recovery as specified above), additional immunotherapy treatments were initiated. Eight posttransplantation immunotherapy treatments were administered at 3-week intervals over a 6-month period (9 total treatments, including the pretransplantation dose).
Study design. Design included leukemia harvest at diagnosis of AML, induction chemotherapy with high-dose cytarabine (Ara-C) and daunorubicin (Dauno), consolidation chemotherapy with high-dose Ara-C and etoposide (VP16), followed by stem cell mobilization with GCSF, peripheral blood stem cell (PBSC) collection, single pretransplantation dose of immunotherapy, followed by collection of primed lymphocytes by lymphapheresis, pretransplantation myeloablative preparative regimen with Busulfan (Bu) and cyclophosphamide (Cy), PBSC and primed lymphocyte infusion, and posttransplantation immunotherapy treatments administered every 3 weeks for 8 doses, beginning at least 6 weeks after transplantation.
Study design. Design included leukemia harvest at diagnosis of AML, induction chemotherapy with high-dose cytarabine (Ara-C) and daunorubicin (Dauno), consolidation chemotherapy with high-dose Ara-C and etoposide (VP16), followed by stem cell mobilization with GCSF, peripheral blood stem cell (PBSC) collection, single pretransplantation dose of immunotherapy, followed by collection of primed lymphocytes by lymphapheresis, pretransplantation myeloablative preparative regimen with Busulfan (Bu) and cyclophosphamide (Cy), PBSC and primed lymphocyte infusion, and posttransplantation immunotherapy treatments administered every 3 weeks for 8 doses, beginning at least 6 weeks after transplantation.
Immunotherapy production and administration
The targeted immunotherapy dose was 108 autologous leukemia cells admixed with 4 × 107 K562/GM cells (secreting GM-CSF at a rate > 500 ng/106 cells/24 hours). Autologous leukemia cells were harvested, processed, irradiated, tested for quality control, cryopreserved, and stored at each clinical site. Leukemia cells were harvested via blood draw, bone marrow aspiration, or leukapheresis to achieve a target of 1-2 × 109 cells. Red blood cells (RBC) were removed by density gradient centrifugation using an established procedure (COBE 2991, DACS device, or equivalent). Assessment of tumor content was done morphologically by Wright-Giemsa staining and confirmed by flow cytometry in most cases. The light density cells were irradiated (10 000 cGy), cryopreserved in autologous plasma and dimethylsulfoxide, and stored frozen in liquid nitrogen.
K562/GM cells, based on the GVAX immunotherapy platform, were derived from an erythroleukemia cell line (K562) modified to secrete GM-CSF, as previously described.22 Clinical lots were prepared under Good Manufacturing Practice conditions (Cell Genesys, Inc), irradiated, cryopreserved, and stored frozen in liquid nitrogen. Six lots of K562/GM were used in the trial with a mean GM-CSF secretion rate of 3008 ng/106 cells/24 hours (range, 2030-4396). Immediately before immunotherapy administration, autologous leukemia cells and K562/GM cells were thawed in a 37°C water bath, mixed together, and administered as multiple 0.5-mL intradermal injections.
Safety and pharmacokinetic assessment
Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria and assessed for relationship to immunotherapy treatment. Time to hematologic engraftment was assessed as a potential marker of autoimmunity directed against hematopoietic stem and progenitor cells. Time to engraftment was measured from ASCT day 0 to ANC > 500/mm3, platelet > 20 000/mm3, platelet > 50 000/mm3, and RBC transfusion independence. Serum GM-CSF levels were monitored in a subset of patients. GM-CSF levels were measured by enzyme-linked immunosorbent assay daily for 4 days at the time of the pretransplantation immunotherapy treatment as well as the first, fourth, and eighth posttransplantation immunotherapy treatments.
Clinical assessment
Patients were monitored for remission status, relapse, and survival. CR was defined as less than 5% bone marrow blasts, resolution of any previously abnormal karyotype, ANC greater than 1000/mm3, platelet count greater than 100 000/mm3, and maintenance of these criteria for at least 30 days. Relapse was defined as greater than 5% bone marrow blasts or recurrence of abnormal karyotype. Time to relapse was measured from the date of first documentation of CR to first documentation of relapse.
Immune response analysis
Anergy panel.
Overall immune responsiveness was assessed using an anergy panel skin test to recall antigens (mumps, Candida, tetanus).
Tumor delayed-type hypersensitivity testing.
Induction of in vivo immune response was assessed by tumor delayed-type hypersensitivity (DTH) skin testing using 2 × 106 autologous leukemia cells per test. Skin test reagents were injected intradermally. Induration of at least 5 mm at 48 to 72 hours was considered a positive reaction.
In vitro antibody response.
Induced antibody responses reactive against autologous leukemia cells and K562/GM cells were analyzed by immunoblot as previously described.13 Detection of new or enhanced intensity bands on immunoblot compared with pretreatment baseline was considered a positive antibody response.
In vitro T-cell response.
Induction of T-cell responses to autologous leukemia cells was performed using an Elispot assay measuring interferon-γ and granzyme-B production (H. Levitsky). A 2-fold increase over pretreatment baseline with at least 5 spots measured was considered a positive reaction. Analysis was limited to patients who received at least 4 posttransplantation immunotherapy treatments. Detailed methods are provided in supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Minimal residual disease monitoring by WT1
The presence of minimal residual leukemia was assessed by monitoring of WT1 transcript levels in blood and bone marrow by using a quantitative RT-PCR assay (W.S.). Detailed methods are provided in supplemental data. Briefly, total RNA was extracted from blood and bone marrow, and cDNA was synthesized using standard techniques. Amplifications of patient samples, K562 cell line cDNA, and no-template controls were performed in triplicate. WT1 expression levels were detected using a transcript-specific primer and probe set. To compensate for differences in RNA integrity and cDNA synthesis efficiency, the absolute WT1 transcript copy number was normalized to the endogenous control gene, ABL. The limit of normalized WT1 transcript quantification was 10−3WT1/ABL. WT1 monitoring was performed at the following time points: enrollment, CR, pretransplantation immunotherapy (days 0 and 14), posttransplantation immunotherapy (treatments 1, 4, and 8), and 3 and 6 months after completion of immunotherapy.
Statistical analysis
It was estimated that approximately 50% of enrolled patients would both achieve CR and proceed to immunotherapy treatment and ASCT, leading to a target of 50 enrolled patients, to yield an evaluable population of 25 immunotherapy-treated patients. RFS and OS were estimated using the method of Kaplan and Meier. Normalized WT1 values recorded as zero were set at 10−4 for the purpose of WT1 log change from baseline analyses. Exploratory multivariate analysis of RFS in the following subgroups was performed: WT1 decline (present or absent), autologous tumor DTH response (< or >5 mm induration), antibody response to autologous tumor or K562/GM cells (present or absent), and cytogenetic risk group. Cytogenetic risk groups were defined as good: t(8:21), inv 16; poor: complex, t(6:9), and del 7; and intermediate: normal, other.27 All authors had access to the primary clinical trial data for review.
Results
Patients and treatment
Fifty-four patients enrolled in the study at 4 investigational sites and underwent leukemia cell harvest; 53 (98%) received induction chemotherapy, and 46 (85%) achieved CR. One patient received dose reduction of daunorubicin (from 60 to 45 mg/m2) during induction chemotherapy, and 5 received an additional induction cycle (cytarabine + anthracycline +/− etoposide) following protocol-specified therapy. Thirty-four patients (63%) received consolidation chemotherapy, all per protocol; 28 (52%) received the first pretransplantation immunotherapy treatment, and 27 (50%) proceeded to ASCT. Twenty-one patients (39%) initiated and 19 (35%) completed all available posttransplantation immunotherapy treatments. Disposition of trial subjects and reasons for study withdrawal are outlined in supplemental Figure 1. The primary reasons for study withdrawal included relapse, death, and triage to alternative therapies. The median time from ASCT to initiation of posttransplantation immunotherapy was 66 days (range, 43-459 days), with delays predominantly due to slow platelet engraftment. Of the 21 patients who received posttransplantation immunotherapy treatments, 5 ultimately relapsed; 3 of these initiated posttransplantation immunotherapy on schedule and 2 were delayed.
Baseline characteristics for all enrolled patients who underwent leukemia cell harvest (n = 54) and those proceeding to pretransplantation immunotherapy (n = 28) are shown in Table 1. Because most of the attrition between enrollment and ASCT occurred among patients with high-risk cytogenetic abnormalities, 75% of immunotherapy-treated patients fell into an intermediate cytogenetic risk group.
Cell processing feasibility
The majority of enrolled patients underwent leukemia cell harvest from blood by either a simple blood draw (41%) or apheresis procedure (52%). The median number of viable leukemia cells harvested was 5.6 × 109 cells (range, 0.73-65.91 × 109); apheresis procedures were, not surprisingly, associated with the highest leukemia cell yields. In only 3 patients (5.6%) was the tumor cell harvest less than 109 cells, the minimum dose required for 9 immunotherapy treatments plus immune monitoring tests. Adequate stem cell harvest (>2 × 106 CD34+ cells/kg) was achieved in 25 of 28 patients (89%), with a median stem cell dose of 6.9 × 106 CD34+ cells/kg. A median dose of “primed” lymphocytes of 1.2 × 108 CD3+ cells/kg (range, 0.7-96 × 108) was collected.
GM-CSF pharmacokinetics
Serum GM-CSF concentrations were monitored after pre- and posttransplantation immunotherapy treatments in 10 patients (see supplemental Table 1). Six patients had detectable GM-CSF levels at one or more time points. A similar pattern was observed after each immunotherapy dose, with peak levels (ranging from 6.1 to 71.1 pg/mL) occurring one day after administration in most patients and measurable levels for up to 4 days. There was no clear evidence of accelerated GM-CSF clearance with repeated immunotherapy administration.
Immune response
Immune response was assessed through anergy panel skin testing, immunotherapy injection site reactions, DTH skin reactions to injections of autologous irradiated leukemia cells, in vitro T-cell response to autologous leukemia cells, and induction of antibody reactivity against autologous leukemia and K562/GM cells. In vivo skin testing results and in vitro T-cell and antibody responses are summarized in Table 2. A response to Candida and mumps was evaluated at multiple time points throughout the study and was present in approximately 50% of patients at any given time point; tetanus reactivity was evaluated at the last posttransplantation immunotherapy treatment only and was present in 29% of patients. In contrast, skin reactions at immunotherapy injection sites were noted in 100% of patients, with at least 94% responding at each time point. DTH reactions to injections of autologous leukemia cells were present in 2 of 26 patients (7.7%) before the first immunotherapy treatment and were induced in 7 of 18 additional patients (39%) at either the fourth or eighth posttransplantation immunotherapy treatment. In each case, induction of a positive tumor DTH response was transient. Six of the 7 patients with induced tumor DTH responses also had responses to Candida, but one had no response to either Candida or mumps.
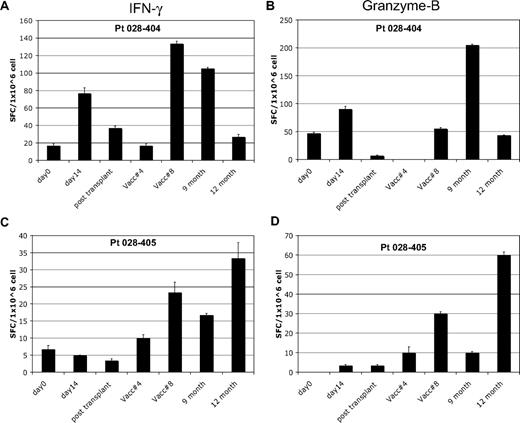
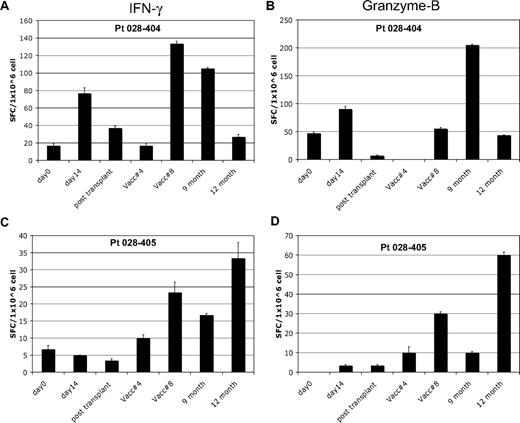
Analysis of in vitro T-cell response to autologous tumor by Elispot was performed after a 7-day in vitro stimulation in patients who completed at least 4 posttransplantation immunotherapy treatments. Seven of 17 patients (41%) showed induction of interferon-γ Elispot responses after the first pretransplantation immunotherapy; 2 of these subjects also showed induction of granzyme-B responses. A representative pretransplantation immunotherapy response is shown in Figure 2A-B. Fifteen of 17 patients showed induction of Elispot responses after posttransplantation immunotherapy, with the majority (10 of 15) demonstrating induction of both interferon-γ and granzyme-B responses. A representative posttransplantation response is shown in Figure 2C-D. Fourteen of these 15 patients remain in continuous CR. All 7 of the patients with induction of autologous tumor skin DTH responses after transplantation also showed induction of in vitro T-cell responses to autologous tumor by Elispot. The peak Elispot response, assessed by either interferon-γ or granzyme-B production, coincided with the time point when DTH conversion was documented in 6 of 7 subjects.
In vitro T-cell immune response to autologous tumor. Results of Elispot assay in 2 representative patients. Blood samples were analyzed at various study time points for T-cell response to autologous leukemia cells and assessed for interferon-γ and granzyme-B production by Elispot. Patient 028-404 showed induction of T-cell response by both interferon-γ (A) and granzyme-B (B) after pre- and posttransplantation immunotherapy. A typical pattern is seen with waning of the pretransplantation response soon after transplantation, followed by recovery with posttransplantation treatments. Patient 028-405 showed induction of T-cell response by both interferon-γ (C) and granzyme-B (D) after posttransplantation immunotherapy only. This response coincided with induction of a tumor DTH skin test response at posttransplantation treatment 8. Analysis time points included: pretransplantation immunotherapy baseline (day 0) and day 14 after the first immunotherapy dose (day 14), before posttransplantation immunotherapy 1 (∼6 weeks after transplantation) and at posttransplantation immunotherapy 4 and 8, and follow-up at month 9 and 12.
In vitro T-cell immune response to autologous tumor. Results of Elispot assay in 2 representative patients. Blood samples were analyzed at various study time points for T-cell response to autologous leukemia cells and assessed for interferon-γ and granzyme-B production by Elispot. Patient 028-404 showed induction of T-cell response by both interferon-γ (A) and granzyme-B (B) after pre- and posttransplantation immunotherapy. A typical pattern is seen with waning of the pretransplantation response soon after transplantation, followed by recovery with posttransplantation treatments. Patient 028-405 showed induction of T-cell response by both interferon-γ (C) and granzyme-B (D) after posttransplantation immunotherapy only. This response coincided with induction of a tumor DTH skin test response at posttransplantation treatment 8. Analysis time points included: pretransplantation immunotherapy baseline (day 0) and day 14 after the first immunotherapy dose (day 14), before posttransplantation immunotherapy 1 (∼6 weeks after transplantation) and at posttransplantation immunotherapy 4 and 8, and follow-up at month 9 and 12.
Induction of antibody reactivity against the immunotherapy components was also assessed. After immunotherapy, 5 of 15 patients (33%) demonstrated induction of antibodies reactive against autologous leukemia cells, whereas 15 of 15 (100%) demonstrated induced antibody reactivity against K562/GM cells. Preliminary analyses in 8 subjects showed no evidence of induction of antibody responses directed against WT1 (data not shown).
Clinical outcome
Forty-six of 53 patients (85%) achieved CR after one or more cycles of induction chemotherapy with a high-dose cytarabine-based regimen. At the time of the primary analysis, median follow-up for surviving patients was 3 years. For all patients who achieved CR, the estimated 3-year RFS rate was 47.4% (95% CI: 30.7%-62.4%) and OS was 57.4% (95% CI: 40%-71.5%). For patients treated with immunotherapy (n = 28), of whom 27 proceeded to ASCT, the estimated 3-year RFS rate was 61.8% (95% CI: 40.2%-77.6%) and OS rate 73.7% (95% CI: 52.5%-86.5%). Limited additional follow-up of the immunotherapy-treated group (median, 5 years) showed an estimated 5-year RFS rate of 55.1% (95% CI: 34.6%-71.6%) and OS of 60.1% (95% CI: 37.7%-76.7%).
Minimal residual disease
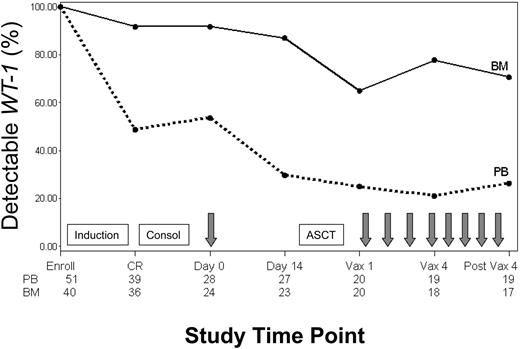
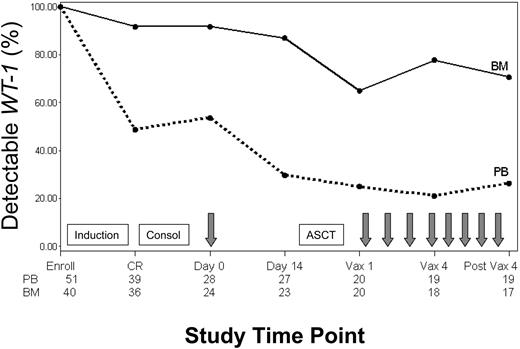
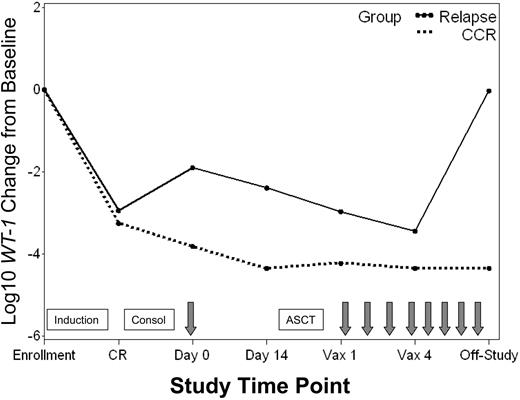
Minimal residual disease (MRD) was monitored in blood and bone marrow throughout the study by using a quantitative molecular assay that measured expression of the gene encoding WT1. The percentage of patients with detectable WT1 transcript levels throughout the study is shown in Figure 3. All patients assessed had detectable WT1 levels in blood and bone marrow at enrollment when overt leukemia was present. More than 90% of patients who achieved CR had associated declines in WT1 in both blood and bone marrow (median of ∼3 logs in blood). Nevertheless, 46% and 95% of patients had persistently detectable WT1 levels in blood and bone marrow, respectively. A change in WT1 transcript copy number in the blood was evaluated between the time of the pretransplantation immunotherapy treatment and collection of the leukapheresis product 2 weeks later. Pretransplantation immunotherapy was delivered a median of 55 days (range, 36-165 days) after initiation of consolidation chemotherapy and 24 days (range, 15-130 days) after stem cell collection. Over this 2-week interval, 11 of 16 patients (69%) with persistently detectable levels of WT1 in blood showed further WT1 declines, with 8 of 11 (70%) achieving an undetectable WT1 status (Figure 3). Of the 3 remaining patients still evaluable for further reductions in WT1 with posttransplantation treatments, one showed a subsequent decline to undetectable levels, one had stable WT1 levels, and one relapsed before initiating posttransplantation immunotherapy. In general, patients who maintained a continuous CR status throughout the study had greater WT1 declines from baseline in both blood and bone marrow than patients who ultimately relapsed (Figure 4).
Detectable WT1 transcript levels in blood and bone marrow. Percentage of patients with detectable WT1 transcript levels in peripheral blood (PB) and bone marrow (BM) at enrollment, CR, pretransplantation immunotherapy day 0 and day 14, posttransplantation immunotherapy 1 (Vax 1) and 4 (Vax 4), and follow-up (post Vax 4). Boxes signify chemotherapy treatments including induction, consolidation (Consol), and ASCT. Arrows signify immunotherapy administration. Numbers along the bottom of the chart signify the number of patients with data at each time point.
Detectable WT1 transcript levels in blood and bone marrow. Percentage of patients with detectable WT1 transcript levels in peripheral blood (PB) and bone marrow (BM) at enrollment, CR, pretransplantation immunotherapy day 0 and day 14, posttransplantation immunotherapy 1 (Vax 1) and 4 (Vax 4), and follow-up (post Vax 4). Boxes signify chemotherapy treatments including induction, consolidation (Consol), and ASCT. Arrows signify immunotherapy administration. Numbers along the bottom of the chart signify the number of patients with data at each time point.
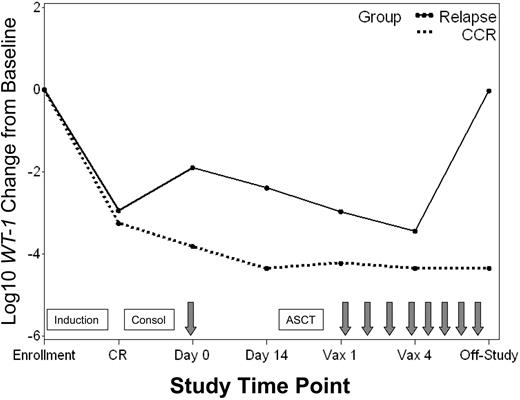
WT1 transcript levels in patients with continuous CR versus relapse. Median log10 change from baseline in WT1 transcript levels for patients who achieved a CR and remained in continuous CR (CCR) throughout the study ( ) versus those who relapsed (–). Time points include: enrollment (enroll), CR, pretransplantation immunotherapy day 0 and day 14, posttransplantation immunotherapy 1 (Vax 1) and 4 (Vax 4), and last study measurement (Off-Study). Boxes signify chemotherapy treatments including induction, consolidation (Consol), and ASCT. Arrows signify immunotherapy administration.
) versus those who relapsed (–). Time points include: enrollment (enroll), CR, pretransplantation immunotherapy day 0 and day 14, posttransplantation immunotherapy 1 (Vax 1) and 4 (Vax 4), and last study measurement (Off-Study). Boxes signify chemotherapy treatments including induction, consolidation (Consol), and ASCT. Arrows signify immunotherapy administration.
WT1 transcript levels in patients with continuous CR versus relapse. Median log10 change from baseline in WT1 transcript levels for patients who achieved a CR and remained in continuous CR (CCR) throughout the study ( ) versus those who relapsed (–). Time points include: enrollment (enroll), CR, pretransplantation immunotherapy day 0 and day 14, posttransplantation immunotherapy 1 (Vax 1) and 4 (Vax 4), and last study measurement (Off-Study). Boxes signify chemotherapy treatments including induction, consolidation (Consol), and ASCT. Arrows signify immunotherapy administration.
) versus those who relapsed (–). Time points include: enrollment (enroll), CR, pretransplantation immunotherapy day 0 and day 14, posttransplantation immunotherapy 1 (Vax 1) and 4 (Vax 4), and last study measurement (Off-Study). Boxes signify chemotherapy treatments including induction, consolidation (Consol), and ASCT. Arrows signify immunotherapy administration.
Examination of RFS in subgroups
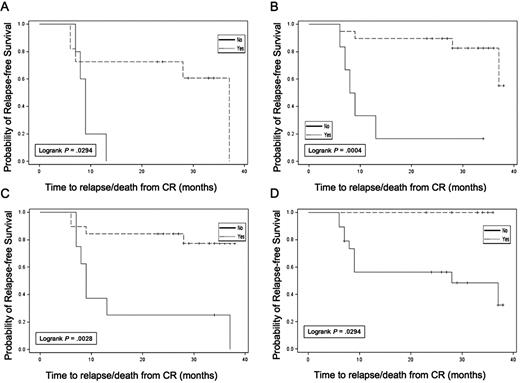
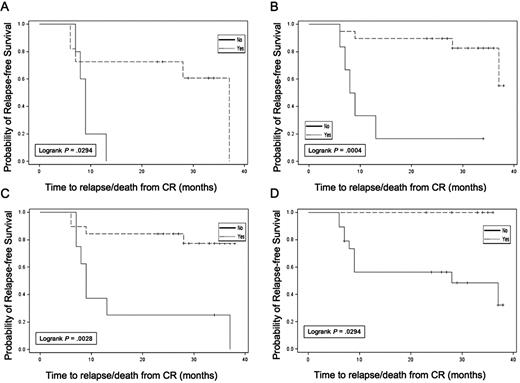
Exploratory analyses were conducted evaluating RFS in subgroups based on WT1 response, autologous tumor DTH response, autologous tumor and K562/GM antibody response, and cytogenetic risk group. Results are summarized in Table 3. Although subgroups were small, limiting data interpretation, several observations were made. Patients with a decrease in WT1 levels in blood after the pretransplantation immunotherapy treatment (Figure 5A) had longer RFS than those without WT1 declines (median, 37.0 vs 9.0 months; P = .029; 3-year RFS, 60.6 vs 0%). In addition, those who achieved undetectable WT1 levels at any time point during the trial (Figure 5B) also had longer RFS (median, 37.0 vs 7.0 months; P = .088; 3-year RFS, 58.2 vs 14.3%). In particular, failure to achieve an undetectable WT1 transcript level before transplantation was associated with a high risk of relapse within 12 months (Figure 5C). In vivo immune response induction was also associated with prolonged RFS; the 7 patients with treatment-induced autologous tumor DTH reactions all remained in continuous CR at last follow-up (3-year RFS, 100 vs 48.1%; Figure 5D). Three of these 7 DTH responders converted from detectable to undetectable WT1 transcript levels after the first immunotherapy dose, and the other 4 had undetectable levels at the onset of immunotherapy. Of the 17 patients analyzed for in vitro T-cell response to autologous tumor, 15 of 17 remain in continuous CR, impeding formal subgroup analysis. However, 14 of 15 of these continuous CR patients demonstrated posttransplantation Elispot responses against autologous tumor. In contrast, no association was seen between induction of new antibody reactivity against autologous tumor and RFS. Finally, cytogenetic risk group was associated with leukemia outcome with a 3-year RFS of 80% in all patients who achieved CR with good risk (n = 7), 51% with intermediate risk (n = 33), and 0% with poor risk (n = 6). Corresponding 3-year RFS data for the 28 immunotherapy-treated patients were 75% with good risk (n = 4), 66% with intermediate risk (n = 22), and 0% with poor risk (n = 2) cytogenetics.
Relapse-free survival in subsets. (A) Reduction in WT1 after pretransplantation dose of immunotherapy. (B) Achievement of undetectable WT1 status at any time during the trial. (C) Achievement of undetectable WT1 status before ASCT. (D) Induction of autologous tumor DTH response (yes,  ; no, –).
; no, –).
Relapse-free survival in subsets. (A) Reduction in WT1 after pretransplantation dose of immunotherapy. (B) Achievement of undetectable WT1 status at any time during the trial. (C) Achievement of undetectable WT1 status before ASCT. (D) Induction of autologous tumor DTH response (yes,  ; no, –).
; no, –).
Safety
No grade 3 or 4 adverse events related to the leukemia cell harvest were reported. During immunotherapy treatment, grade 1 or 2 injection site reactions, reported in all patients, were the most common adverse event. Other common related adverse events included pain, pruritis, and headache (each 21%), fatigue (18%), urticaria, and feeling abnormal (each 14%). Only one related grade 3 event (arthralgia) was reported. Due to a theoretical concern about induction of autoimmunity against the hematopoietic system by the pretransplantation dose of immunotherapy and subsequent reinfusion of “primed” lymphocytes with the stem cell graft, time to posttransplantation hematologic engraftment was monitored. The median time to ANC > 500/mm3 was 15 days (range, 13-21), to platelets > 20 000/mm3 was 12 days (range, 6-108), and to platelets > 50 000/mm3 was 20 days (range, 9-435). A median of 3 platelet transfusions (range, 0-41) and 3 RBC transfusions (range, 0-22) were administered after transplantation.
Discussion
This trial explored the combination of ASCT, adoptive transfer of immunotherapy-primed lymphocytes, and pre- and posttransplantation immunotherapy based on the GVAX platform as postremission therapy for adult AML. The results demonstrate the feasibility of immunotherapy production, a favorable toxicity profile, and encouraging signals of potential activity. These signals included induction of tumor DTH skin reactions, antitumor T-cell and antibody responses, and reductions in minimal residual disease as measured by WT1 transcript levels. Furthermore, clinical outcomes were at least comparable with historical single center data evaluating ASCT as postremission therapy in AML.2
More than 1 billion leukemia cells (median, 5.6 billion) were successfully collected at diagnosis in 95% of enrolled patients, with the majority obtained through a simple blood draw or apheresis procedure. The tumor cell yield was higher, with tumors harvested through less invasive procedures than in previous clinical trials evaluating this mixed GVAX platform in nonsmall cell lung cancer19 and multiple myeloma.21 In the nonsmall cell lung cancer trial, the median tumor cell dose harvested via surgical procedures was only 40 million cells. In the myeloma trial, the median tumor cell dose harvested was 3.5 billion cells but required a high-volume bone marrow harvest under general anesthesia. Clearly, acute leukemia represents an optimal clinical setting in which to harvest autologous tumor cells for immunotherapy applications.
In a subset of patients, serum GM-CSF levels were monitored as a potential biomarker of K562/GM pharmacokinetics. As observed in previous trials,19,21 the majority of subjects showed reproducible levels of serum GM-CSF after repeated dosing. K562 cells, based on their lack of human leukocyte antigen expression, are targets of natural killer cell cytotoxicity,22 but this did not result in rapid in vivo clearance. Furthermore, peak levels of serum GM-CSF were below that reported to result in significant induction of myeloid suppressor cells and inhibition of immunogenicity in animal studies.28
Immunotherapy was well tolerated with expected toxicities of injection site reactions and flu-like symptoms. No patients suffered from engraftment failure, a theoretical risk of this immunotherapy approach and protocol design. Time to neutrophil engraftment (median, 15 days) was somewhat delayed compared with most published studies using mobilized peripheral blood stem cells (median, 9-10 days),2 and delayed platelet engraftment was observed in some patients. Delayed neutrophil and platelet engraftment have been reported in patients receiving autologous CD34+ stem cell doses of less than 10 million cells, with a median time to ANC greater than 500/mm3 of 12 days and platelets greater than 50 000/mm3 of 46 days reported in this population.29 The CD34+ stem cell dose administered in this study was less than 10 million cells in the majority of patients (median, 6.9 million), making this a potential explanation for delayed engraftment. However, an effect of immunotherapy, particularly on neutrophil engraftment, remains possible.
Treatment-associated immune responses were demonstrated and included induction of autologous tumor DTH skin reactions, in vitro T-cell responses to autologous tumor by Elispot, and autologous tumor-reactive antibodies. Of interest is that all 7 patients with positive DTH reactions postimmunotherapy have remained relapse-free, with a minimum follow-up of 2 years. Furthermore, while the analysis was skewed toward patients still in CR after completion of 4 posttransplantation immunotherapy treatments, 14 of 15 such patients demonstrated measurable Elispot responses to autologous tumor after transplantation. The transient nature of the induced tumor DTH responses may have been due to clearance of leukemia antigens in the setting of continuous CR and/or cessation of immunotherapy treatment. In most subjects, the time point of tumor DTH reactivity coincided with peak levels of Elispot reactivity. At least one prior trial using the GVAX platform in the adjuvant setting in the treatment of resected pancreatic cancer also showed a positive association between autologous tumor DTH reactions and freedom from relapse.15
Monitoring of minimal residual disease in leukemia trials is a powerful tool that has been shown to be highly predictive of response to therapy and clinical outcome in multiple hematologic cancers, including acute promyelocytic leukemia (PML-RARα by RT-PCR),30,31 chronic myeloid leukemia (bcr-abl by RT-PCR),32,33 and acute lymphoblastic leukemia (various methods).34,35 WT1 codes for a zinc-finger transcription factor that is overexpressed in the majority of acute leukemias, making this an attractive pan-leukemia cell marker for monitoring of MRD across the entire karyotypic spectrum of AML.24,25 Previous studies have demonstrated an association between persistent or recurrent detection of WT1 in patients with AML in CR and risk of relapse.26,36 Cilloni et al recently reported that detectable WT1 in the blood of AML patients in first CR was a significant adverse prognostic factor, even when adjusted for other risk factors or treatments, including ASCT. In their series of 71 AML patients who entered a CR after induction therapy, 23 had persistently measurable WT1 in the blood and all 23 went on to relapse.26
In our study, all patients had detectable levels of WT1 in both blood and bone marrow at study entry. Nevertheless, clearance of WT1 from the blood was only achieved in approximately half of the patients in CR, even after one additional round of consolidation chemotherapy, suggesting that the amount of additional leukemia cell kill achieved with repeated cycles of high-dose cytarabine-based chemotherapy is minimal. After receipt of a single dose of immunotherapy pretransplantation, however, two-thirds of patients showed a further decline in WT1 transcript levels in blood, with 70% of patients achieving undetectable levels. The ability to monitor further declines in WT1 after posttransplantation treatments was, therefore, limited. A decline in WT1 after the first immunotherapy dose was associated with longer relapse-free survival. In fact, all patients without a measured WT1 decline in response to pretransplantation immunotherapy went on to relapse within one year of transplantation. It is unlikely that a late effect of prior consolidation chemotherapy accounted for the declines in blood WT1 levels observed after this first treatment given that: (1) the median 3-log reduction in WT1 levels after induction was observed within 6 weeks of initiation of chemotherapy, (2) no incremental reduction in median WT1 levels in blood was observed 8 weeks after consolidation chemotherapy, and (3) the pretransplantation immunotherapy was administered at a time when normal hematopoiesis had recovered from the cytotoxic effects of chemotherapy. It is also unlikely that clearance of mobilized WT1-expressing hematopoietic progenitors from the blood after stem cell collection contributed to this measured WT1 decline, given that mobilization of CD34+ progenitors into the blood typically declines to background levels within several days of cessation of GCSF mobilization37 and that stem cells were collected a median of 24 days before the first dose of immunotherapy in this trial.
In general, subjects whose leukemia ultimately relapsed had higher levels of WT1 postremission than subjects who remained in continuous CR. Furthermore, achievement of undetectable WT1 levels after induction or consolidation chemotherapy, immunotherapy, or ASCT was associated with favorable outcome, suggesting that clearance of MRD, as measured by WT1 transcript levels, may be a useful biomarker to follow in the management of AML, in general, and in the conduct of trials evaluating postremission therapies in patients in hematologic remission, in particular.
Although background WT1 expression in normal bone marrow progenitors likely accounted for persistent detection of WT1 transcripts in the marrow in the majority of patients throughout the study, expression of WT1 in normal blood is rare. Therefore, persistent expression in blood likely represents true MRD.24,25 This makes peripheral blood not only the most convenient but also the best source material for WT1 monitoring of MRD in AML.
In conclusion, this study demonstrated encouraging signals of activity that were positively associated with clinical outcome in younger patients with AML in the setting of ASCT. These data suggest that this immunotherapy approach may lead to significant reductions of MRD in the majority of patients with leukemia in remission. Furthermore, given the rapid WT1 response noted after a single dose of immunotherapy before ASCT, it would be reasonable to explore this and other platforms of leukemia-specific immunotherapy as postremission maintenance therapy in the absence of ASCT, especially in older patients with AML for whom aggressive postremission therapies may not be indicated.
The online version of this article contains a data supplement.
This study was presented in part at the 2005 Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, May 14, 2005.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Ferdynand Kos and Allan Hess of the Johns Hopkins Human Immunology Core Laboratories for their assistance in the in vitro T-cell assays.
The study was supported by research funding from Cell Genesys, Inc, South San Francisco, CA. Additional correlative research support was provided to Dr Wendy Stock for analysis of WT1 and to Dr Hyam Levitsky for analysis of immune response. Preclinical development was supported by National Institutes of Health 2P01CA15396-23 (H.L.).
National Institutes of Health
Authorship
Contribution: I.M.B. designed research, performed research, analyzed data, and edited the paper; H.I.L. designed research, performed research, contributed analytical tools, analyzed data, and edited the paper; W.S. designed research, performed research, contributed vital analytical tools, analyzed data, and edited the paper; D.S. contributed vital analytical tools and analyzed data; L.Q. contributed vital analytical tools, and performed research; D.J.D., E.P.A., R.M.S., L.E.D., and C.A.L. designed research, performed research, and edited the paper; D.J.M. analyzed data; and K.M.H. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: K.H. and D.M. are past employees and stockholders of Cell Genesys, Inc. Under a licensing agreement between Cell Genesys and the Johns Hopkins University, H.I.L. and I.M.B. are entitled to a share of milestone payments and a share of royalties received by the university on sales of GVAX. H.I.L. previously served as a paid consultant to Cell Genesys. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. W.S. and H.I.L. received funding to support correlative studies, including analysis of WT1 and immune monitoring, respectively. The remaining authors declare no competing financial interests.
Correspondence: Hyam Levitsky, MD, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, 1650 Orleans St, Room 4M51, Baltimore, MD 21231; e-mail: hy@jhmi.edu.
References
Author notes
*I.M.B. and H.I.L. contributed equally to this work.


 ) versus those who relapsed (–). Time points include: enrollment (enroll), CR, pretransplantation immunotherapy day 0 and day 14, posttransplantation immunotherapy 1 (Vax 1) and 4 (Vax 4), and last study measurement (Off-Study). Boxes signify chemotherapy treatments including induction, consolidation (Consol), and ASCT. Arrows signify immunotherapy administration.
) versus those who relapsed (–). Time points include: enrollment (enroll), CR, pretransplantation immunotherapy day 0 and day 14, posttransplantation immunotherapy 1 (Vax 1) and 4 (Vax 4), and last study measurement (Off-Study). Boxes signify chemotherapy treatments including induction, consolidation (Consol), and ASCT. Arrows signify immunotherapy administration.