Abstract
Abstract 914
NFAT (Nuclear factor of activated T cells) are ubiquitous proteins that play a major role in regulating inducible gene expression in the immune system, and comprise important biological functions in a variety of other organs. Stimulation of the T cell receptor (TCR) coupled to calcium mobilization triggers activation of many intracellular enzymes including calcineurin, which dephosphorylates and activates NFAT. Upon activation, NFAT proteins translocate from the cell cytoplasm to the nucleus where they transcribe a large array of activation-associated genes (Rao, 1997). In T cell development, NFAT activation is a key regulator for thymocyte development and positive selection (Aifantis, 2001; Neilson, 2004).
Concurrent in vivo imaging of migration and activation of T cells has not been previously described. We developed a dual-reporter system enabling the determination of NFAT activation status (by NFAT-inducible Click-beetle luciferase) in addition to the cell distribution (by constitutively expressed membrane-anchored Gaussia luciferase). We applied this system to T cells and T cell precursors in murine studies of graft versus host disease (GVHD) and immune reconstitution respectively. We recorded spatial-temporal differences between their migration and NFAT activation by bioluminescence imaging (BLI).
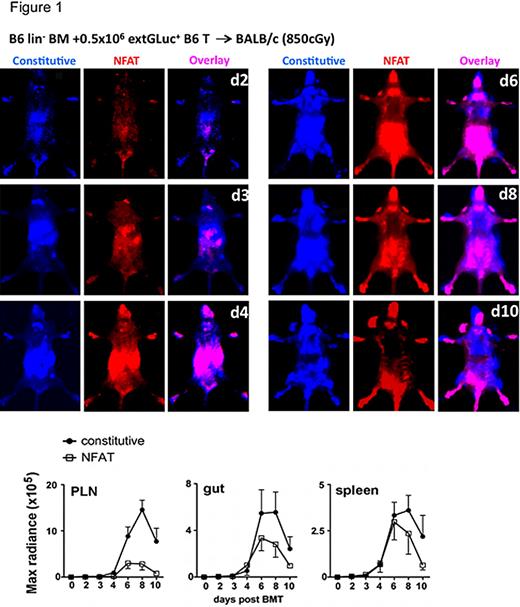
To assess T cell activation in the setting of GVHD, we transplanted lethally irradiated mice with lineage-depleted bone marrow (lin- BM) and donor T cells. In the very early post-BMT phase, donor T cells migrated to both peripheral lymph nodes (PLN) and the intestines, whereas the NFAT activation was dominant in the intestines. Subsequently, PLN showed the highest constitutive BLI signal, but had the lowest NFAT-activation to constitutive signal ratio (CBRLuc-to-extGLuc). This observation suggests either a lower activation per cell or a relatively small percentage of activated cells in PLN. Interestingly, both the constitutive and induced signals from the alloreactive T cells dropped precipitously after day 8 post transplantation. This decline of alloreactive T cells might represent a contraction phase involving activation-induced cell death (AICD) (Russell, 1995). NFAT signaling can be related to sustained alloactivation and regulation of AICD of T cells since both the CD95 ligand and TNFa promotor contain multiple NFAT binding sites (Rao, 1997) (Figure 1).
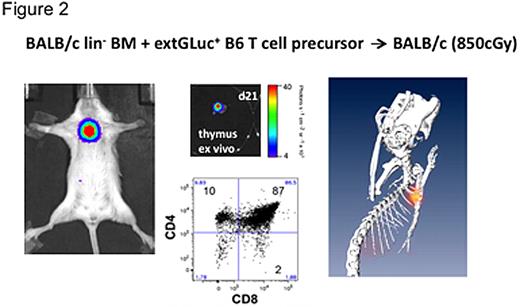
To assess the development and selection of T cell precursors, we adoptively transferred CD4-CD8-CD25+CD44+ (DN2) and CD4-CD8-CD25+CD44- (DN3) T cell precursor cells into lethally irradiated recipients of allogeneic lin- BM cells. First NFAT-inducible activity (CBRLuc BLI) was detectable in the thymus on days 11-14 after transfer of transgenic T cell precursors, which were derived from OP9-DL1 coculture of transduced LSK cells. We also acquired BLI data in a manner suitable for tomographic reconstruction and calculated the three-dimensional distribution of the NFAT-activation signal. The result clearly showed a localization of activated cells in the region of the thymus, which was confirmed by subsequent ex vivo assays (Figure 2).
We used the HY transgenic mouse model (Kisielow, 1988) to analyze the predominant negative versus positive selection of thymocytes in male and female mice, respectively. When we adoptively transferred transgenic HY-TCR DN2/DN3 T cell precursors into B6 (H-2b) hosts of both sexes, we could detect transferred T cell precursors in the thymi of both male and female mice, but NFAT signaling and development into CD4+ or CD8+ single-positive (SP) cells could be detected only in the thymus of female mice. Based on previously published data (Teh, 1990), we suggest that the lack of detectable SP cells and NFAT signaling in male mice is due to early deletion of T cell precursors by negative selection, either concomitant or preceding the generation of CD4+CD8+ cells.
To our knowledge we describe for the first time the successful application of a dual constitutive and NFAT-inducible reporter system suitable for studies of primary cells in small animal models. As NFAT proteins play important roles in immune and non-immune cells, the reporter system could be applied in a wide range of cell types, including studies of immunological tolerance, cell differentiation and carcinogenesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.