Abstract
Abstract 510
Recently, Redaelli et al (J Clin Oncol. 2009;27:469) compared the in vitro inhibitory activity of imatinib, dasatinib, nilotinib, and bosutinib against 18 mutant forms of BCR-ABL (expressed in transfected Ba/F3 cells) associated with imatinib resistance and proposed a chart to assist in the selection of second-generation tyrosine kinase inhibitors (2TKIs) for the treatment of imatinib-resistant CML associated with mutations. However, the predictability of this chart has neither been clinically evaluated nor does it take into account other important clinical factors (e.g. pharmacokinetics (PK)/pharmacodynamics) that may impact response rates to 2TKIs in the presence of mutations. The purpose was to assess the impact of 2TKIs' in vivo plasma levels on the in vitro GI50 data, and to determine if in vitro GI50 data with or without plasma levels correlates with observed clinical responses in imatinib-resistant patients (pts) with mutations.
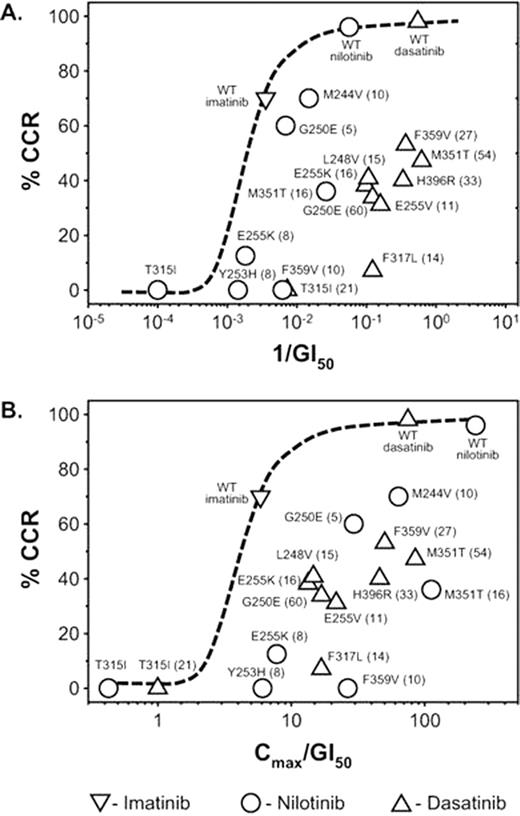
To enable appropriate comparison of the activity of 2TKIs against specific mutations we modified the original in vitro GI50 data by adjusting it to include an estimate of in vivo Cmax exposure data for each 2TKI. Further refinement was achieved by calculating the Cmax/GI50 values for each agent and normalizing these against imatinib vs wild-type BCR-ABL. To assess the correlation between patient response and in vitro GI50 data, the previously published CCyR rates for pts with specific mutations were plotted according to in vitro GI50 values alone and against the adjusted Cmax/GI50 values.
The adjusted Cmax/GI50 data suggest that nilotinib delivers the most potent inhibition of most BCR-ABL mutations in vivo, with dasatinib being the next most potent. However, when either in vitro GI50 data alone or the modified Cmax/GI50 data are considered, there is poor correlation of clinical responses to both nilotinib and dasatinib against several of the mutations in vivo (Figure). Overall, activity of 2TKIs against all mutations was less than expected based on original in vitro GI50 or Cmax/GI50 calculations of systemic exposure. For example, the G250E mutation has similar systemic exposure to nilotinib as the F359V mutation as indicated by Cmax/GI50, but substantial differences are observed in the CCyR rate (60% vs 0%). For dasatinib, the same was observed for the F317L and L248V mutations which have similar exposures to dasatinib but have different CCyR rates (7% vs 41%). Similarly, several mutations with comparable exposure to nilotinib and dasatinib had substantial differences in CCyR rates, suggesting that other factors were influencing responses. For example, the G250E mutation was considered moderately sensitive to both nilotinib and dasatinib based on the adjusted Cmax/GI50; however, CCyR rates on nilotinib were much higher (60%) compared with dasatinib (34%). Similarly, the E255K mutation was considered moderately sensitive to both agents based on the adjusted Cmax/GI50; however, CCyR rates on dasatinib were much higher (38%) compared with nilotinib (13%).
This analysis illustrates the limitations of in vitro inhibition data alone or in combination with PK exposure data in the selection of 2TKI therapy for imatinib-resistant pts with mutations. The current analysis still does not consider parameters such as protein binding and intracellular influx/efflux, among a variety of other clinical factors that could further influence response rates. This tool is also not useful for pts with mutations of unknown in vitro sensitivity, which may represent 15% of all pts with mutations. Currently, clinical responses remain the best approach for selection of 2TKIs in pts with mutations, with only a small subset of mutations having low sensitivity mutations existing for each 2TKI. Other factors, such as patient medical history, comorbidities, and the agents' safety profiles, are also important in selection of 2TKIs.
Laneuville:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Wyeth: Research Funding; ChemGenex: Research Funding. DiLea:Novartis: Employment. Mestan:Novartis: Employment. Yin:Novartis: Employment, Equity Ownership. Woodman:Novartis: Employment. Manley:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.