Abstract
Abstract 4282
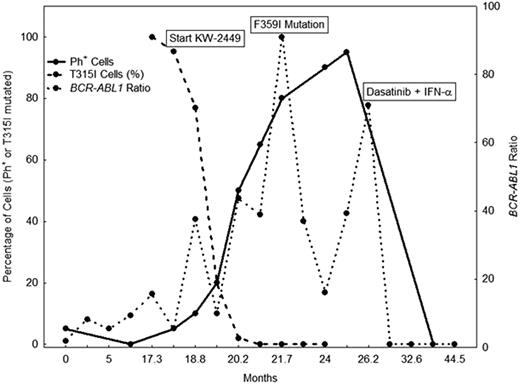
Mutations of BCR-ABL1 have been observed in 50% of patients with chronic myeloid leukemia (CML) who develop resistance to imatinib. The gate-keeper mutation T315I is one of the mutations with universal resistance to imatinib and to the second-generation tyrosine kinase inhibitors (TKI) that are approved for the treatment of patients with imatinib failure. The use of new kinase inhibitors with in vitro activity against T315I mutation as well as other agents with different mechanisms of actions is being evaluated in clinical trials. We report the case of a 57-year old man that was diagnosed with CML in 2003. Patient received initial therapy with standard-dose imatinib that was subsequently increased to 800 mg daily. He did achieve a complete cytogenetic response (CCyR) 9 months post dose escalation. He was followed by RT-PCR for BCR-ABL1.. In May, 2007, the patient BCR-ABL1/ABL1 ratio increased to 16.38 but the patient remained in CCyR. BCR-ABL1 sequencing revealed the T315I mutation in 100% of cells (Figure 1). One month later the patient lost CCyR (5% Philadelphia-positive [Ph+] cells) and the BCR-ABL1/ABL1 ratio was 5.08. The patient was started on the T315I specific inhibitor KW-2449 (100 mg orally twice daily for 14 days, every 3 weeks). Patient had a progressive decline in percentage of cells with the T315I mutation (Figure 1). However, at the same time he had an increase in percentage of Ph+ cells. In September, 2007, three months after starting therapy with KW-2449, patient had no cytogenetic response (80% Ph+ cells, PCR for BCR-ABL1 ratio > 100) and the T315I mutation was undetectable. At that time, a new ABL1 sequencing revealed the F359I mutation (no quantification was done). Patient was maintained on KW-2449 for the next 6 months, without significant improvement in cytogenetic response nor BCR-ABL1 ratio, but the clone with the T315I mutation did not reappear. In February, 2008, the patient lost hematologic response and presented with an elevated white blood cell count of 22×109/L. The F359I mutation was still present. Therapy with KW-2449 was stopped and the patient started dasatinib 100 mg/day and Interferon-a 3,000,000 units. Three months later, the patient acheived CCyR with a BCR-ABL1/ABL1 ratio of 0.05. At the last evaluation, 16 months after the start of dasatinib and interferon combination, the patient was maintaining CCyR and major molecular response. In conclusion, this case illustrates the benefit of the use of combination therapy, mainly TKI and agent with different mechanism of action either sequentially (TKI followed by KW-2449) or concomitantly (TKI + interferon) in eradicating resistant disease with T315I clone.
Serial Monitoring of Ph+ Cells, T315I Cells and BCR-ABL1/ABL1 Ratio
Serial Monitoring of Ph+ Cells, T315I Cells and BCR-ABL1/ABL1 Ratio
Cortes:Novartis: Research Funding. Jabbour:Novartis: Speakers Bureau; Bristol Myers Squibb : Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.