Abstract
The most common subtypes of primary cutaneous T-cell lymphomas are mycosis fungoides (MF) and Sézary syndrome (SS). The majority of patients have indolent disease; and given the incurable nature of MF/SS, management should focus on improving symptoms and cosmesis while limiting toxicity. Management of MF/SS should use a “stage-based” approach; treatment of early-stage disease (IA-IIA) typically involves skin directed therapies that include topical corticosteroids, phototherapy (psoralen plus ultraviolet A radiation or ultraviolet B radiation), topical chemotherapy, topical or systemic bexarotene, and radiotherapy. Systemic approaches are used for recalcitrant early-stage disease, advanced-stage disease (IIB-IV), and transformed disease and include retinoids, such as bexarotene, interferon-α, histone deacetylase inhibitors, the fusion toxin denileukin diftitox, systemic chemotherapy including transplantation, and extracorporeal photopheresis. Examples of drugs under active investigation include new histone deacetylase inhibitors, forodesine, monoclonal antibodies, proteasome inhibitors, and immunomodulatory agents, such as lenalidomide. It is appropriate to consider patients for novel agents within clinical trials if they have failed front-line therapy and before chemotherapy is used.
Introduction
Primary cutaneous lymphomas are composed of both T-cell (75%+) and B-cell lymphomas and are rare conditions representing 2% of all lymphomas with an annual incidence of 0.3 to 1 per 100 000.1,2 There are a variety of different types of cutaneous T-cell lymphoma (CTCL); and until relatively recently, there were 2 classifications for CTCL, the World Health Organization (WHO)3 and the European Organization for Research and Treatment of Cancer (EORTC),4 the latter characterized by dividing the entities into aggressive or indolent conditions based on clinicopathologic criteria. In 2005, the 2 classification systems were combined (Table 1). In this review, we focus on the most common forms of CTCL, mycosis fungoides (MF) and its leukemic variant, Sézary syndrome (SS).
The wide array of clinical presentations and possible treatment modalities makes the treatment of MF/SS complex, and there are no simple treatment algorithms. There are several published guidelines, which we recommend the reader review, that provide more detail around the rationale of our management approaches to the various presentations of MF/SS. These include the National Cancer Center Network guidelines (www.nccn.org)5 and those by the European Society of Medical Oncology6 and the EORTC,7 with our approach most closely reflecting the latter. It is, however, very important to recognize that these guidelines are based on a somewhat restricted evidence base as CTCLs are very rare diseases with very few randomized trials performed to date. Moreover, when planning treatment, individual patient factors need to be considered, such as age and comorbidities, especially the risk of infection for which patients with MF/SS are particularly prone. The management approach is truly multidisciplinary; and, as such, we hope to provide the combined perspectives of a dermatologist, radiation oncologist, and hematologist-oncologist. A summary of the various treatment options we generally consider are outlined in Table 2 and, in this review, we aim to address the most common clinical scenarios the clinician faces.
Investigations
It cannot be overemphasized that the diagnosis of CTCL requires clinicopathologic correlation, and review by a pathologist colleague experienced in these disorders is strongly recommended. A consensus approach to diagnosis of early-stage MF has been recently reported by the International Society of Cutaneous Lymphoma (ISCL) with the majority of cases of CTCL diagnosed on hematoxylin-and-eosin sections with appropriate immunophenotyping, most commonly by immunohistochemistry and in some cases by flow cytometry and clonal T-cell receptor gene rearrangement by polymerase chain reaction on fresh and formalin-fixed tissue.8 The approach to diagnosis is summarized in Table 3 and uses an algorithm integrating clinical and laboratory assessments.
It is also important to recognize that it is not uncommon for the diagnosis of MF to remain elusive for many years, often requiring observation and repeated biopsies.9,10 Such an approach avoids embarking on numerous investigations in a disease that is generally indolent and where outcome is not altered by aggressive early intervention.
Non-MF cutaneous T-cell lymphoma
A key aspect of the management of CTCL is to distinguish the rare non-MF CTCL entities from MF.1 Clinical presentation will often help distinguish them from MF, and clinicopathologic correlation is critical to distinguish MF from other rarer CTCL subtypes, transformed disease, peripheral T-cell lymphoma or perhaps even a rare variant of MF. The approach to management of non-MF CTCL is highly variable ranging from a conservative approach with CD30+ CTCL, such as lymphomatoid papulosis (LyP), to a very aggressive approach in such conditions as cutaneous γ/δ T-cell lymphoma or primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. It is beyond the scope of this review to discuss the non-MF CTCL entities in detail, and we refer the reader to the WHO-EORTC manuscript for an overview of the clinical presentation and treatment strategies.1
Stage
The management of MF/SS is centered on a “stage-based” approach, and MF is classified into 4 clinical stages based on the TNM classification (Table 4), which then is synthesized into a clinically based staging system broadly divided into early- and advanced-stage disease11 (Table 5). Skin patches and plaques occur in stage I, which is divided into IA (< 10% body surface area [BSA]) or IB (≥ 10% BSA). The presence of clinically evident lymphadenopathy without pathologic nodal infiltration represents stage IIA, cutaneous tumors characterize stage IIB, generalized erythroderma characterizes stage III, and pathologically positive lymph nodes (IVA) and visceral disease characterizing stage IVB. Patients with staged IA, IB, and IIA disease are considered to have “limited-stage” disease, and those with stages IIB (tumor), III (erythroderma), and IV (pathologic nodes with or without viscera) have “advanced-stage” disease.
Prognosis
Although MF/SS are generally considered incurable conditions, it is important to recognize that the majority of patients have an indolent form of the disease and will live for many years. Indeed, it is estimated that 65% to 85% of patients with MF have stage IA or IB disease.9,12 The most important factor in planning management and determining prognosis is the stage of the disease. Indeed, the majority of patients with early-stage disease (stages IA, IB, and IIA) do not progress to more advanced-stage disease, and patients presenting with isolated patch or plaque disease (T1-T2) have a median survival of more than 12 years12-14 (Figure 1). Moreover, patients with stage IA disease do not appear to have a decreased survival compared with an age-, sex-, and race-matched population.13
Actuarial disease-specific survival of 525 patients with MF and SS according to their clinical stage at diagnosis (stages IA-IV).12 For stage IA versus IB disease, P = .007; for stage IB versus IIA disease, P = .006; for stage IIA versus IIB disease, P < .001; for stage IIA versus III disease, P = .03; for stage IIB versus III disease, P = .09; and for stage IA-III versus IV disease, P < .001.
Actuarial disease-specific survival of 525 patients with MF and SS according to their clinical stage at diagnosis (stages IA-IV).12 For stage IA versus IB disease, P = .007; for stage IB versus IIA disease, P = .006; for stage IIA versus IIB disease, P < .001; for stage IIA versus III disease, P = .03; for stage IIB versus III disease, P = .09; and for stage IA-III versus IV disease, P < .001.
Patients with advanced-stage disease (stages IIB, III, and IVA) with tumors, erythroderma, and lymph node or blood involvement but no visceral involvement have a median survival of 5 years from time of presentation. Of note, patients with tumors (T3) have an inferior outcome to those with erythroderma (T4). Patients with visceral involvement are rare (stage IVB) and have a median survival of only 2.5 years or less.9,12,13,15,16
Investigations.
The approach to staging the patient is summarized in Table 6 and based on the recommendations of the ISCL.11 For patients with clinically very limited-stage disease with skin patches and/or plaques with no palpable lymphadenopathy, extensive staging investigations are not generally required. Occasional patients will present with locoregional lymphadenopathy, which may reflect dermatopathic changes in the node rather than true nodal involvement with MF. Thus, it is not always necessary to biopsy every patient with mildly enlarged nodes. In general, we recommend biopsy of nodes larger than 1.5 cm as nodal involvement has substantial prognostic impact (Table 4). The relative hesitancy in performing node biopsies relates to the high incidence of skin colonization with pathogenic organisms in patients with MF/SS, which increases the risk of infection after surgery.
Prognostic characteristics beyond stage.
Clinical stage is by far the most important predictor of outcome. However, within early-stage MF, there is some prognostic heterogeneity. Indeed, we recognize an “intermediate-risk” group between early- and advanced-stage disease. This includes patients with stage IIA/IB folliculotropic variant of MF and patients with very thick plaques.17,18 The relatively inferior outcomes in these groups are thought to be the result of its reduced responsiveness to skin-directed therapy (SDT).19 For advanced-stage disease, patients with stage IIB disease with multiple tumor nodules (a higher tumor burden) and large-cell transformation of MF have a substantially poorer prognosis (see “Transformed disease”).9 Low numbers of CD8+ T cells in the dermal infiltrate and/or the blood have also been independently associated with reduced survival.14,20,21
Managing early-stage (IA-IIA) MF
Overview
As mentioned, the majority of patients present with early-stage disease (Table 7). As the use of early application of therapy does not impact on survival,16 a nonaggressive approach to therapy is warranted with treatment aimed at improving symptoms and cosmesis while limiting toxicity. As patients with stage IA disease have a long life expectancy, an “Expectant Policy” may be a legitimate management option in selected patients, provided that it incorporates careful monitoring. Given that multiple skin sites are often involved, the initial treatment is primarily SDT, which aims to control skin lesions while minimizing morbidity. The key choices for SDT are topical or intralesional corticosteroids or psoralen plus ultraviolet A radiation (PUVA) or ultraviolet B (UVB). Indeed, for patients with limited patch disease, topical steroids often control the disease for many years, and often this is the only form of therapy required for such patients. Patch and thin plaque MF can be treated with topical corticosteroids. Class I (potent) topical corticosteroids, such as betamethasone dipropionate 0.05% or mometasone furoate 0.1%, are the most effective at obtaining objective disease regression. Patients with stage T1 disease have an approximately 60% to 65% complete response (CR) rate and a 30% partial response (PR) rate with topical steroids. Patients with T2 disease (generalized patch/plaque with > 10% of skin surface involved) have a 25% CR rate and a 57% PR rate. Topical corticosteroids have CR rates similar to other forms of SDTs.22 Intralesional corticosteroids can be effective in treating thicker MF lesions, such as plaques or tumor deposits.
For more widespread disease, phototherapy with PUVA or UVB is recommended. Response rates to PUVA therapy in patients with patch disease are high with CR rates of approximately 58% to 83% and overall response rates of up to 95%.23,24 Furthermore, remission is often prolonged with a reported mean duration of 43 months.23 Maintenance treatment with weekly or fortnightly therapy can be effective in maintaining remission. PUVA therapy is generally well tolerated; however, acute side effects include nausea (from the oral psoralens) or photosensitivity. Long-term side effects are acceleration of actinic damage and an increased rate of skin malignancies, including squamous cell carcinoma and melanoma.25-27
UVB is also effective for MF, especially for patch and thin plaque disease. Broadband UVB (300-320 nm) was initially used, and more recently narrow band UVB (311 nm) has also been shown to be effective in MF, although remission duration with the latter may be inferior. The advantage of UVB over PUVA is that it is more readily available (more community-based dermatology practices have UVB equipment) and avoids the need for protective sunglasses and the side effects, albeit modest, of psoralen. The disadvantage of UVB is its somewhat lower response rate and duration of remission and less effective than PUVA with thicker lesions.28,29 PUVA has been reported to achieve improved response rates when combined with interferon-α-2b (IFN-α)30,31 or retinoids such as acitretin.32 PUVA therapy has also been used as a salvage or maintenance therapy after total skin electron beam (TSEB) therapy.33 For even thicker plaques, particularly if localized, radiotherapy is effective as the disease is highly radiosensitive (see “Radiotherapy”).
Other choices for first-line therapy are topical chemotherapy using mechlorethamine (nitrogen mustard [NM]) or carmustine. However, the use of these agents can be impractical if lesions are extensive and, with long-term use, carry a risk of secondary epidermal cancer. Moreover, particular care must be taken to avoid topical exposure to those carers assisting with the application of the solution or ointment. Drug hypersensitivity is reported to occur in up to 45% or more of patients treated with topical NM, particularly in solution form. NM ointment reduces the incidence of allergic reactions; however, it involves considerable pharmacy preparation and consequently is not readily available. Skin sensitivity occurs in up to 5% of patients treated with carmustine. Other localized therapies include imiquimod34 and photodynamic therapy,24 but the latter is limited to specialized centers.
“Second-line” therapy for early-stage disease is often retinoids or rexinoids (bexarotene), IFN-α, low-dose oral methotrexate (MTX), histone deacetylase inhibitors (HDACi), or denileukin diftiox. Such second-line therapy can be highly effective for disease refractory to topical therapies, and these choices are always considered before the use of chemotherapy. Radiotherapy is a highly effective therapy in MF/SS and can be used for both early- and advanced-stage disease, as first-line or relapsed/progressive disease.
Radiotherapy
Cutaneous lymphomas are usually highly radiosensitive, and radiation therapy may play a major role in the management of many patients with MF.35 Partial regression of disease may be observed with single doses as low as 1.0 Gy.36 However, permanent eradication of all disease using radiotherapy alone is an elusive goal. Thus, treatment is usually aimed at improving symptoms and cosmesis. Nonetheless, there is the very occasional patient who presents with truly localized MF (single lesion) often around the “bathing trunk” distribution or breast. Whether this is curable is unknown, but our approach is similar to the management of other low-grade lymphomas: to treat such patients with local radiotherapy with “curative” intent to a dose of approximately 30 Gy. A large proportion of these patients may remain disease-free.37
The likelihood of achieving a CR and the durability of those responses decreases with increasing stage of disease; patients with T1 disease have a more than 80% CR rate with radiotherapy (either local field or TSEB therapy), compared with 20% to 30% CR rates for T4 disease. Five-year relapse-free survival rates with radiation alone are 40% to 60% for T1 disease, but less than 10% for T4 disease.37 Irrespective of stage and curability, however, radiotherapy can provide excellent palliation of troublesome symptoms of MF/SS, such as pruritus, scaling, and ulceration.
Target volume.
For most patients, the target volume is the epidermis and/or dermis, that is, the maximum depth of interest is only a few millimeters from the skin surface unless there are tumors or deep ulcers. Most lesions may therefore be treated with very soft (low penetrance) beams: superficial x-ray therapy (50-145 kvp) or 4 to 9 MeV electron beams. Higher-energy beams (orthovoltage/megavoltage) are occasionally necessary for thicker lesions.
TSEB therapy is usually reserved for patients with extensive skin involvement and can be used as first- or second-line therapy for patients with extensive T2 or T3 disease, occasional patients with T4 disease, and those who are no longer responding to topical therapies. Even when the responses are incomplete or the duration of complete response is brief, patients usually achieve significant clinical benefit. It is a complex technique and requires the use of either multiple field arrangements or a rotational technique, with “patching” or “boosting” for areas of underdosing and self-shielding (eg, soles of feet, perineum) and takes 6 to 10 weeks to complete.
Dose.
A wide range of radiation doses may be used in the management of these patients. For symptomatic treatment of individual lesions, the dose may even be titrated to the response and usually 15 to 20 Gy is sufficient. Although very small doses of radiation can provide effective palliation of these lesions, there does appear to be a dose-response relationship for complete remission, especially in the context of TSEB therapy. Doses of 10 to 20 Gy are associated with a CR rate of only 55%, whereas doses of 30 Gy or greater are associated with a 94% CR rate. In addition, the durability of responses is greater for patients treated with higher doses.38 The maximum dose that is tolerated in a single course of TSEB is approximately 36 Gy, beyond which there is significant acute toxicity.
Combined modality treatments.
For patients with extensive and/or resistant disease, radiation has been used sequentially with several other treatments: PUVA, UVB, retinoids, and topical or systemic chemotherapy. Occasionally, treatments may be administered concurrently, but doses of radiation will have to be modified if large fields are being treated to minimize the risk for erythema or desquamation. Extreme modifications to the radiation schedule and lengthy treatment breaks may compromise the effectiveness of the radiotherapy. TSEB followed by adjuvant PUVA, NM, photopheresis, or other adjuvants does lead to a significant benefit in disease-free survival, but not in overall survival (OS).39,40 One combined modality approach for patients with extensive disease that we have found to have promising efficacy is the use of 2 or 3 courses of chemotherapy, eg, high-dose MTX (> 1 g/m2) or liposomal doxorubicin to reduce disease to clinically minimal levels before proceeding with TSEB.
Patient factors.
Many patients with MF/SS are in good general health and may be working full-time. Others may be elderly or not reside close to a center that offers TSEB therapy. In either case, a 10-week course of treatment may not be feasible and other management options will have to take precedence. Coexisting medical problems rarely preclude a patient from radiotherapy, but there are some contraindications, eg, scleroderma, or inability to stand for several minutes at a time during TSEB therapy.
Retinoids and rexinoids
Retinoids belong to the family of steroid hormones, which bind to the nuclear receptors (retinoic acid receptor [RAR]; retinoid X receptor [RXR]) and subsequently interact with various transcription factors. RAR and RXR have various isoforms (α, β, and γ), which are differentially expressed in tissues. The skin contains both RAR and RXR. Non–RXR-selective retinoids, such as oral etretinate, arotinoid, acitretin, and isotretinoin (13-cis-retinoic acid), have been used alone or in combination with PUVA, IFN-α, or even chemotherapy and are reported to have response rates in the range of 5% to 65%.31,32,41-45 Bexarotene is a new synthetic rexinoid that selectively binds to the RXR subfamily and is formulated as either as capsule or a topically applied gel.46,47 In our experience, oral bexarotene can achieve responses in chemoradiotherapy refractory patients within 2 to 4 months, and those patients may have a sustained benefit provided that the RXR-induced hyperlipidemia is manageable, allowing an optimal therapeutic dose. Bexarotene may also be useful in maintaining responses after SDT.46,48-50 Topical bexarotene is particularly useful for patients who have a limited number of patches or plaques, and we recommend its use before topical chemotherapy.47 In general terms, bexarotene is being used more frequently in MF/SS, often in place of the earlier generation retinoids.
IFN-α and related biologic response modifiers
IFN-α, a biologic response modifier, should generally be considered as second-line therapy for stage IA-IB disease and a first-line therapy for IIB, III, and SS and is effective at moderately high doses of 3 million to 10 million units (MU) daily or 3 times/week.51-53 Time to response is in the order of weeks, and it can be combined with PUVA, chemotherapy, retinoids, and bexarotene.30,31,41,42,46,50,54,55 In advanced-stage disease, our preference is to use single-agent IFN-α first, adding PUVA if there is more widespread pruritus and adding bexarotene if the response is suboptimal. Prolonged responses have also been observed with γ-interferon.56 Recombinant interleukin-12 (IL-12) has efficacy in MF, but limited availability does not make it a realistic treatment option at present.57
Low-dose MTX
There are few published reports on the use of MTX in MF,58,59 with the largest series of 60 patients with patch/plaque MF (T2) achieving a 12% CR and 22% PR rate with a median time to treatment failure of 15 months.58 In this study, the median weekly dose was 25 mg with maximum doses up to 75 mg. Low-dose MTX has been successfully combined with IFN-α.60
Clinical case 1: early-stage disease and SDT
Scenario
A 42-year-old woman living in large metropolitan city presented with stage IB MF with predominantly cutaneous patches on the trunk involving 40% of the BSA (Figure 2).
Management
The patient commenced PUVA therapy 3 times/week for 6 months with complete resolution of lesions. PUVA was continued for a further 3 months, twice a week and then discontinued. Localized lesions returned (∼ 5% of BSA) on her trunk 40 months later. By that time, the patient had moved 140 miles from the city. PUVA was not used because of inconvenience and a limited extent of cutaneous disease. Topical corticosteroids were used initially with good response for 3 years. When more extensive lesions with plaques developed, the patient was treated successfully with IFN-α 3 MU daily with complete resolution of symptoms. At the time of next progression, we will consider retreatment with PUVA (if convenient) with or without oral bexarotene or IFN-α.
Comment
This case highlights the durable effect of SDT and how therapy options need to be individualized.
Clinical case 2: early-stage disease and SDT for “intermediate-prognosis” disease
Scenario
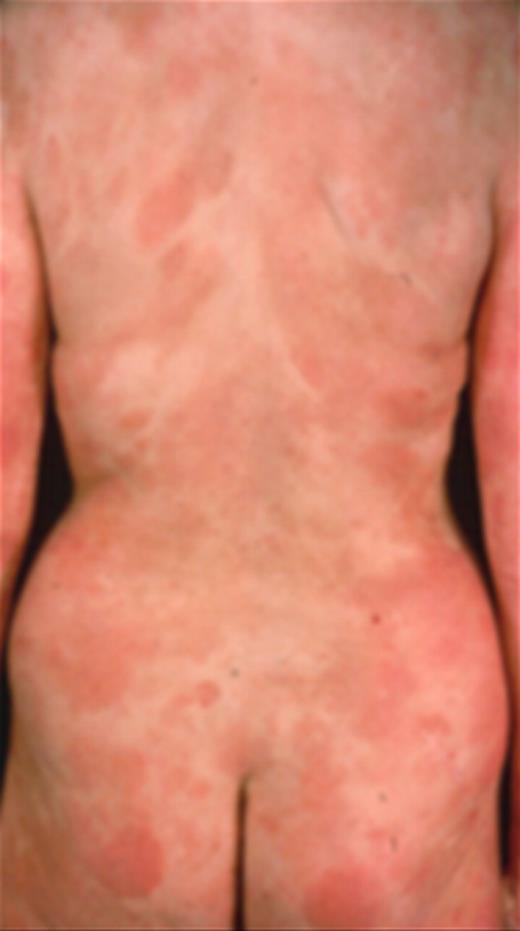
A 65-year-old patient presented with multiple patches and plaques (> 50% of BSA), some showing clinical and histologic evidence offolliculotropism. Peripheral nodes in the inguinal and axillary areas were palpable with CT scan confirming that the largest axillary node measured 2.5 cm. A nodal biopsy revealed dermatopathic features confirming stage IIA disease (Figure 3).
Patient with stage IB disease with folliculotropic plaques on the trunk.
Management
The patient was treated with PUVA phototherapy twice weekly for 4 months with only a limited partial clinical response. IFN-α was started (initially 3 MU 3 times/week, increasing to 6 MU 3 times/week) while the patient continued PUVA for a further 3 months. Prominent thick plaques involving facial areas and the inner thighs were treated with local superficial radiotherapy with complete resolution. The patient's disease responded with a good PR and resolution of peripheral lymphadenopathy after a further 3 months of therapy. Further superficial radiotherapy was used successfully to treat residual disease in the inguinal areas (shielded from PUVA). PUVA was discontinued after further maintenance weekly therapy for 2 months (cumulative UVA dose [550 J/cm2]). Unfortunately, despite continuing IFN-α (3 MU 3 times per week) as maintenance therapy, the patient's disease relapsed within 4 months. Therefore, PUVA was restarted with bexarotene; but after a further 6 months of therapy, only a PR was achieved. Higher doses of bexarotene were not tolerated, and the cumulative dose of PUVA (> 850 J/cm2) was considered a relative contraindication to maintenance PUVA therapy in view of the known carcinogenic potential with high cumulative doses (> 1200 J/cm2). Therefore, the patient was treated with TSEB with a sustained complete remission.
Comment
This case illustrates what we could consider an intermediate prognostic disease in a patient with folliculotropic disease and dermatopathic nodal disease. Patients who are partially resistant to SDT often do well with combined modality treatment, especially PUVA plus IFN-α/bexarotene combinations with additional use of local skin radiotherapy for resistant plaques. The response duration to TSEB will be critical as extensive subsequent relapse, even with early cutaneous stages of disease, will represent a difficult management issue.
Advanced-stage (IIB-IVB) MF
Overview
Treatment of advanced-stage disease, or indeed refractory early-stage disease, is more problematic and always requires a multidisciplinary approach. Although systemic multiagent chemotherapy is often considered in patients with advanced-stage disease, the randomized National Cancer Institute study demonstrated that combination chemoradiotherapy offered no survival benefit over “conservative” sequential therapy.16 Moreover, relatively rapid relapses are observed after chemotherapy; consequently, SDT or biologic response-modifying agents should be used first where practicable and systemic chemotherapy considered in patients progressing after these treatments. Critically, these patients will often have resistant or relapsed disease characterized by only cutaneous patches and plaques, which will require SDT rather than a traditional escalation of systemic therapy. The choice of systemic therapy depends largely on age, performance status of patient, tempo of the disease, risks of myelosuppression, and most importantly, stage. Thus, our approach is to separately consider treatment options of patients with stage IIB (Table 8), stage III/SS (Table 9), stage IV (Table 10), and transformed disease. In general, IFN-α, bexarotene, vorinostat, and the fusion toxin denileukin diftitox are generally considered before embarking on systemic chemotherapy. Conversely, for the relatively rare patient with stage IVB disease of suitable performance status, aggressive chemotherapy, including transplantation strategies, should be considered early. Novel agents within clinical trials should always be considered in these patients. The single-agent or multiagent chemotherapy regimens described in Table 11 are selected depending on disease characteristics and side-effect profile of the agents. The value of extracorporeal photopheresis (ECP) is generally limited to patients with erythrodermic disease and circulating malignant cells (see “SS”).
Denileukin diftitox
Denileukin diftitox is a recombinant targeted fusion protein that combines the receptor-binding sequence of IL-2 with the cytotoxic A-chain and translocation B-chain of diphtheria toxin (DAB389IL-2).69-71 This drug has recently been approved by the Food and Drug Administration in the United States for patients with relapsed CTCL whose tumors express the IL-2 receptor subunit (CD25). This approval is based on superior outcomes in the first placebo-controlled randomized trial of systemic therapy in MF/SS.72 The response rate was 49.1% at the 18 μg/kg dose with no statistically significant difference in RR in patients with early- or advanced-stage disease. Moreover, some patients had prolonged remissions with the median progression-free survival beyond 971 days in the 18 μg/kg arm. This benefit needs to be balanced against a toxicity profile that includes capillary leak syndrome, fever, and fluid retention, and so this is likely to remain a second- or third-line therapy. Recent evidence indicates that durable responses are also seen in patients with CD25− disease.73 It has been successfully combined with bexarotene.74
HDACi
Histone deacetylase inhibitors have activity in various hematologic malignancies, including myeloid malignancies, Hodgkin lymphoma, peripheral T-cell lymphoma, and CTCL.75,76 Vorinostat (suberoylanilide hydroxamic acid) is an orally available hydroxamic acid derivative that inhibits both class I and II histone deacetylases and has been approved in the United States by the Food and Drug Administration for the treatment of relapsed and refractory CTCL.77 In the initial phase 2 study, there was an overall response rate of 24%, with a reduction in pruritus seen in 58% of patients.78 In a subsequent trial, a 30% RR was observed in patients with stage IIB or higher disease.79 The most common toxicities are gastrointestinal or constitutional symptoms, hematologic abnormalities, or taste disorders, and are usually of mild to moderate severity and typically manageable.80 Other HDACi in development, such as romidedpsin (depsipeptide),81 panobinostat,82 and belinostat,83 have demonstrated responses in MF/SS.
Monoclonal antibodies
Alemtuzumab, the humanized monoclonal antibody targeted against CD52w (a pan-lymphocyte antigen) has demonstrated efficacy in MF/SS; however, patients on trials to date have generally been very heavily pretreated, which have probably impacted on the relatively short duration of response and the substantial cytomegalovirus reactivation and hematologic toxicity observed.84-88 Trials of combination strategies in less-heavily pretreated patients are warranted. In general, outside the clinical trial setting, it has a very limited place in the treatment of MF/SS. Hopefully, more T cell–specific antibodies will be developed with less immunosuppressive effects. For example, zanolimumab (HuMax-CD4) is a fully humanized anti-CD4 monoclonal antibody and is specific for the CD4 receptor expressed on most T lymphocytes. Although the antibody interferes with T-cell activation, infections are uncommon. Single-agent response rates are more than or equal to 50%, but remission duration is relatively short.89 Combination studies would be interesting but, to our knowledge, are currently not being investigated in CTCL.
Systemic chemotherapy
Several chemotherapy agents have demonstrated activity in MF/SS. We refer the reader to a detailed and comprehensive review of systemic chemotherapy in CTCL (Table 11).90 In brief, systemic agents include alkylating agents (cyclophosphamide, chlorambucil), anthracyclines, purine analogs, and etoposide. Whereas single-agent or combination chemotherapy regimens have produced moderately high response rates in patients with advanced-stage MF/SS, these responses are typically not durable. There is no recognized superior multiagent chemotherapy regimen for MF, and regimens that are typically associated with the treatment of B-cell lymphoma or Hodgkin lymphoma, such as those using cyclophosphamide, vincristine, vinblastine, prednisolone, MTX, doxorubicin, or mechlorethamine, have a disappointing track record in MF/SS. For example, a study of infusional EPOCH (etoposide, vincristine, doxorubicin, bolus cyclophosphamide, and oral prednisone) in advanced refractory MF/SS demonstrated an overall response rate of 80% with 27% CRs61 ; however, the median duration of response was just 8 months (range, 3-22 months). This study also highlighted the problem of infectious complications in the delivery of chemotherapy in patients whose disease renders them inherently immune-suppressed and who are frequently colonized with potentially pathogenic bacteria.91,92 The combination therapy of cyclophosphamide, MTX, etoposide, dexamethasone alternating with doxorubicin, bleomycin, and vinblastine is suitable for selected younger patients and has demonstrated a 5-year disease-free survival of 27%.42,62
Because of the high risk of infection and myelosuppression and modest response durations with combination chemotherapy, single-agent therapies are preferred, except in patients who are refractory or who present with extensive adenopathy and/or visceral involvement or constitutional symptoms and require rapid tumor reduction. Thus, in patients with relatively slowly progressive disease who have failed other treatments, we would consider low-dose oral MTX, chlorambucil, cyclophosphamide, or etoposide. For patients with more rapidly progressive disease and of reasonable performance status, our preference is to use single-agent gemcitabine,63,93-95 pentostatin,54,64,96-100 or liposomal doxorubicin65,101,102 as these agents have been investigated the most thoroughly. Gemcitabine has a high response rate, but myelosuppression can be problematic with dose-reduction frequently required. A recent report suggests that patients with CTCL may be more prone to other nonhematopoietic toxicities of this drug.95 Overall response rates as high as 70% have been reported for pentostatin with a variety of regimens used. However, such high response rates are generally observed in patients with SS with lower response rates expected in patients with tumor stage or nodal disease. Infectious complications can be reduced with prophylactic trimethoprim and antiviral therapies.64 It has been combined with IFN-α with improvement in progression-free survival. Single-agent fludarabine has a poor response rate of less than 20%, but one combination strategy that appears promising is fludarabine with cyclophosphamide (Table 11). However, short- and long-term hematologic toxicity can be problematic.66
Transplantation
Interpretation of the transplantation data are difficult because the number of patients with MF/SS treated to date with stem cell transplantation is very small. A review of this subject has recently been published.103 In brief, standard-intensity allogeneic stem cell transplantation has been shown to induce complete and durable remissions in patients with CTCL; however, infection rates are high.104-106 Investigators have been evaluating reduced-intensity conditioning for MF/SS and transplantation-related mortality is low, time to relapse is variable, and durable remissions are observed.107,108 We recommend that allogeneic transplantation be considered in younger patients with advanced-stage disease if not responding to agents such as IFN-α, bexarotene, HDACi, or denileukin diftitox.
Results with autologous stem cell transplantation have not been particularly promising.103,109 However, despite the limited data, in younger patients with stage IIB, stage IV, or transformed disease who are refractory/relapsed after IFN-α, bexarotene, or HDACi, their outcome is extremely poor and aggressive approaches seem warranted.5 High-dose therapy has the potential to increase response, and it has been our observation that, although virtually all patients relapse, some patients relapse with somewhat more indolent disease that in turn is more easily managed with nonchemotherapy agents. Clearly, more investigation is required for this group of patients.
Novel agents within clinical trials
In the last few years, several new agents have become available for the treatment of MF. These include bexarotene, denileukin diftitox, and vorinostat. For patients who are not suitable or fail these drugs, novel agents within clinical trials should always be considered. Indeed, it is our belief that patients should be considered for clinical trials as an alternative strategy to systemic chemotherapy. Novel agents that are being investigated in the context of clinical trials are listed in Table 12.
Clinical case 3: progressive disease despite SDT
Scenario
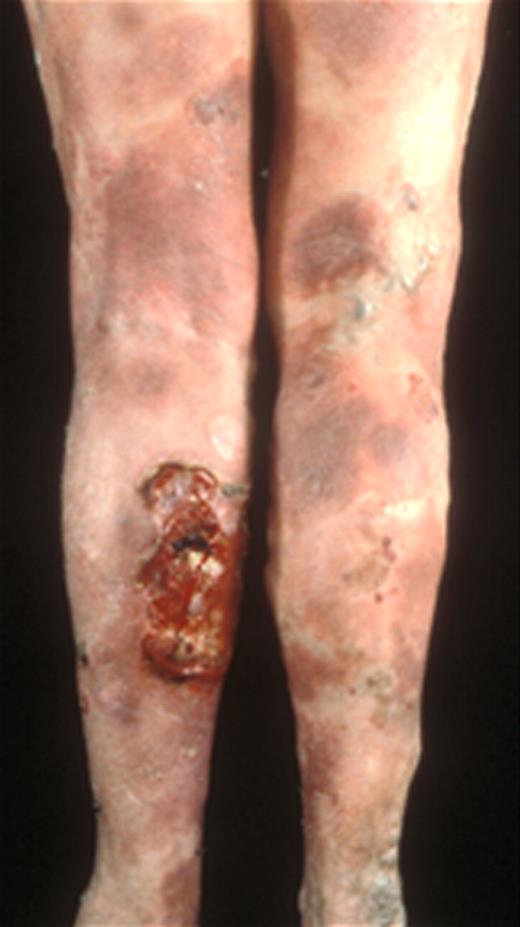
A 52-year-old male patient presented with an 18-month history of polymorphic patches and plaques involving the limbs and pelvic girdle area, which had been partially controlled with topical steroids and attributed to “psoriasis.” A diagnosis of MF was made on the basis of diagnostic clinical and pathologic features (stage IB; Figure 4).
Management
Phototherapy was initiated with initial success, but the patient developed an ulcerated tumor on the right calf, which histologically showed scattered large blastlike cells (CD30−). A staging CT scan was normal. The tumor responded to radiotherapy and PUVA was continued with an excellent response, allowing withdrawal of therapy after 5 months. The patient remained well for 5 years despite developing on average 2 small- to medium-sized tumors every 18 months, which responded to further radiotherapy. However, a recurrence of extensive cutaneous patches and plaques was noted to have gradually developed at routine clinic review, and examination revealed a bulky axillary node (3 cm), which histologically showed partial effacement with malignant lymphocytes. A CT scan showed no other abnormality and the patient (with now “stage IVA disease”) was treated with CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone). An initial complete response after 2 cycles was followed by a recurrence of extensive cutaneous disease (characterized by patches/plaques and scattered tumors) after 4 cycles despite complete resolution of the nodal disease. Chemotherapy was discontinued, the patient was entered into a clinical trial, and a donor search for reduced-intensity conditioning allogeneic transplantation was initiated.
Comment
This case emphasizes the clinical heterogeneity of MF and the challenge of deciding when to escalate therapy as some patients with advanced but limited disease (stage IIB) can be successfully managed with SDT, including radiotherapy, whereas extensive skin tumors (stage IIB) or nodal disease (stage IVA) invariably require aggressive therapies, including chemotherapy and consideration of high-dose therapies, including allogeneic transplantation if the patient's performance status is satisfactory.
Transformed disease
Although most patients with early-stage disease (patches or plaques confined to the skin) have an indolent course, progression to cutaneous tumors, nodal, or visceral disease can occur. Cutaneous tumors can develop either as increasing depth of the small atypical lymphocytes of MF or as a result of large-cell transformation. Thus, when a patient presents with a tumor nodule, it is critical to biopsy the lesion as treatment and outcomes for advanced-stage disease versus transformed disease are quite different (Figure 5). Large-cell transformation is currently defined as large cells (≥ 4 times the size of a small lymphocyte) in more than 25% of the infiltrate or if these cells formed microscopic nodules.118,119 There is a variable incidence of transformation from MF of 8% to 39% reported, and it is associated with a very poor prognosis with a median survival of less than 2 years with particularly short survival in those patients who transform early after diagnosis. The risk of transformation is associated with advanced-stage, elevated β2-microglobulin and elevated lactate dehydrogenase9,118 (Table 13).
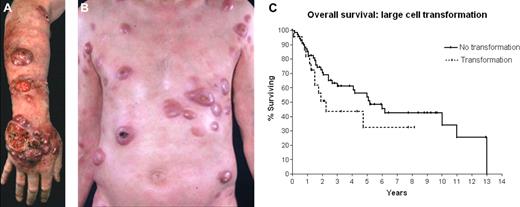
Patient with MF with transformed disease. (A) On limbs. (B) On trunk. (C) OS of patients with advanced-stage disease according to the presence (n = 22) or absence (n = 70) of large-cell transformation in the advanced-stage population.9 Median OS in the transformed group was 2.2 years, compared with 5.2 years in the nontransformed group.
Patient with MF with transformed disease. (A) On limbs. (B) On trunk. (C) OS of patients with advanced-stage disease according to the presence (n = 22) or absence (n = 70) of large-cell transformation in the advanced-stage population.9 Median OS in the transformed group was 2.2 years, compared with 5.2 years in the nontransformed group.
Treatment of transformed disease is a major challenge as these patients generally have a poor outcome. Prognosis is particularly poor when patients have multiple sites of large-cell transformation. There are limited preliminary data to indicate that some patients with advanced-stage disease in whom the large cells express CD30 may have a more indolent course.9,120 For the younger patient, systemic chemotherapy is initiated early and consideration should be made for autologous or allogeneic transplantation. Consolidative radiation therapy should be considered in young patients with unifocal transformation. In elderly or frail patients with unifocal disease, local radiation therapy should be used and occasionally may result in durable remissions.
Clinical case 4: transformed disease—is it or is it not?
Scenario
This 40-year-old woman presented with a 2-year history of erythematous patches and plaques over the pelvic girdle area and limbs, which was initially considered to be eczema by her local medical officer. She then presented with a 2-month history of small tumors on the trunk. A diagnostic biopsy showed an infiltrate of large CD30+ anaplastic cells and staging investigations were normal, suggesting a diagnosis of primary cutaneous CD30+ anaplastic lymphoma. However, a biopsy of the “eczematous” rash showed an epidermotropic infiltrate of CD4+ T cells consistent with MF. In addition, closer discussion with the patient revealed a history of recurrent nodules and small tumors, which always resolved to leave varioliform scars. The diagnosis was changed to MF (stage IB) with LyP. Identical T-cell clones were detected in biopsies from the patches/plaques and the tumor (Figure 6).
Patient with patches and plaques of MF (stage IB) on the limbs and self-healing papules and nodules of lymphomatoid papulosis on the trunk.
Patient with patches and plaques of MF (stage IB) on the limbs and self-healing papules and nodules of lymphomatoid papulosis on the trunk.
Management
The presenting tumor was treated with local radiotherapy, and the patient achieved a complete response to PUVA phototherapy after 4 months of therapy. Subsequently, she remained off therapy with only topical emollients, but frequent recurrences of self-healing papules and nodules prompted successful maintenance treatment with low-dose oral MTX.
Comment
This case illustrates the critical need for expert clinical and pathologic correlation to make the correct diagnosis and avoid inappropriate aggressive therapy. MF is often associated with LyP, and treatment/prognosis is based on the stage of MF. Such patients have to be distinguished from those patients with MF who develop disease progression characterized by tumors with large-cell transformation (dermal sheeted nodules of large pleomorphic or anaplastic blast-like cells, which may or may not be CD30+). Moreover, we think that the current pathologic criteria for large-cell transformation in MF are inadequate and need to be further clarified to aid in distinguishing the various entities.
Sézary syndrome
SS is currently defined by the ISCL as a distinctive erythrodermic CTCL with hematologic evidence of leukemic involvement121 (Figure 7). The WHO-EORTC considers SS to be a separate entity from cases that otherwise meet the criteria for SS but have been preceded by clinically typical MF.1,122 Such latter cases have been designated as “SS preceded by MF” and also as “secondary” SS.123 Patients with SS can be classified either as stage III or IV. The median survival of classic SS in one report of 62 patients was 31 months with a 5-year survival of 34%.124 It appears that the overall prognosis of SS/erythrodermic MF (E-CTCL) is improving; and in a recent report of 124 patients with E-CTCL, there was a median OS of 5.1 years (range, 0.4-18.6 years).125 When patients were stratified according to Sézary cell (SC) counts, the median OS was 7.6 years for patients with less than 1000 SC/L versus 2.4 years for those with more than 10 000 SC/L. In multivariate analysis, advanced age and elevated lactate dehydrogenase were the strongest predictors of a poor prognosis.125 In another study of 106 patients with erythrodermic MF, median survival ranged from 1.5 to 10.2 years depending on the presence of 3 independent adverse factors: patient age, presence of lymph node disease, and peripheral blood involvement.126
Patients with E-CTCL present a difficult management problem because they often have severe itch and a high risk of infection complicating therapy, and remission durations after therapy are frequently short. In general terms, the treatment is similar to that of advanced-stage MF. Systemic therapy or TSEB is often required if PUVA therapy fails. However, patients with erythroderma are exquisitely sensitive to radiation therapy, and planned doses may have to be reduced. In patients with E-CTCL, it is always important to consider underlying staphylococcal infection “driving” disease exacerbation. Indeed, it is well recognized that recognition and treatment of underlying infection (which is often not clinically evident) will result in clinical improvement in the patient's erythroderma.92,127
One treatment that is more effective in E-CTCL compared with other stages of MF is ECP. The efficacy of ECP in E-CTCL has been reviewed elsewhere, and these reviews are highly recommended.128-130 In brief, phase 2 studies have reported a therapeutic benefit of ECP in CTCL, although the response data have been variable, ranging from 30% to 80% depending on study entry criteria, patient selection, and intervals between diagnosis and treatment. No phase 3 (randomized) trials have been performed.
In line with the United Kingdom consensus guidelines, we recommend that patients with E-CTCL with circulating lymphocytes (molecular, flow, or morphology) have an initial trial with ECP.130 The regimens used are variable131 but in general require an intense induction and then maintenance phase.130 Responses may take 6 months or more. Improved RR has been reported when used in combination with bexarotene, PUVA, and IFN-α. In general, we recommend combining ECP with bexarotene or IFN-α.
Second-line therapy for E-CTCL is detailed in Table 9. Patients with E-CTCL have been well represented in clinical trials of bexarotene, denileukin diftitox, HDACi, and alemtuzumab; and indeed, in several trials patients with erythroderma appear to have a better outcome than nodal or tumor-stage disease with such strategies. Chemotherapy, including transplantation strategies, should be considered in younger patients. Single-agent chemotherapy regimens, such as gemcitabine or pentostatin, are our preferred chemotherapy choices (Table 11).
Summary
The first step in managing MF/SS is obtaining an accurate diagnosis, which always requires good communication between the clinician and pathologist. In some instances, close observation and repeated biopsies may be needed. Treatment requires an individualized approach largely depending on the stage of disease and performance status of the patient. Overaggressive therapy with multiagent chemotherapy should be avoided, particularly in patients with early-stage MF/SS. Exciting novel targeted therapies are under investigation, which will add to the armamentarium of treatments for this challenging group of diseases.
Authorship
Contribution: H.M.P., S.W., and R.T.H. wrote the paper.
Conflict-of-interest disclosure: H.M.P. is a consultant to Merck, Novartis, Gloucester Pharmaceuticals, Eisai, and has received research funding from Merck, Novartis, and Gloucester Pharmaceuticals. S.W. is a consultant for Gloucester Pharmaceuticals, Eisai, and Merck. R.T.H. declares no competing financial interests.
Correspondence: H. Miles Prince, Division of Haematology and Medical Oncology, Peter MacCallum Cancer Institute, Locked Bag 1, A'Beckett St, Melbourne 8006, Victoria, Australia; e-mail: miles.prince@petermac.org.