Abstract
This report evaluates the spatial profile of blood vessel fragments (BVFs) and CD34+ and CD117+ hematopoietic stem and progenitor cells (HSPCs) in human cancellous bone. Bone specimens were sectioned, immunostained (anti-CD34 and anti-CD117), and digitally imaged. Immunoreactive cells and vessels were then optically and morphometrically identified and labeled on the corresponding digital image. The distance of each BVF, or CD34+ or CD117+ HSPC to the nearest trabecular surface was measured and binned in 50-μm increments. The relative concentration of HSPCs and BVFs within cancellous marrow was observed to diminish with increasing distance in the marrow space. On average, 50% of the CD34+ HSPC population, 60% of the CD117+ HSPC population, and 72% of the BVFs were found within 100 μm of the bone surfaces. HSPCs were also found to exist in close proximity to BVFs, which supports the notion of a shared HSPC and vessel spatial niche.
Introduction
Quantifying the spatial profile of blood vessels and primitive cell populations most susceptible to myelosuppression or hematologic toxicity is of major interest in basic cancer research, bone marrow transplantation, and molecular radiotherapy.1-3 Although several groups have investigated the spatial location of hematopoietic stem and progenitor cells (HSPCs) in murine models,4-7 evidence for corresponding HSPC spatial profiles within human bone marrow has only recently been established.8 In their study, Watchman et al used novel methods for the digital quantification of the CD34+ hematopoietic stem cells and blood vessel fragments (BVFs) in human iliac crest.8 The present study uses similar techniques of immunohistochemistry and digital image processing to directly measure the concentration of BVFs and CD34+ HSPCs as a function of distance from the most proximal trabecular surface in human bone marrow. The study further advances the findings of Watchman et al, however, through (1) immunohistochemical stratification of the additional population of CD117+ hematopoietic stem and progenitor cells, (2) consideration of a possible bone-site dependence of the BVF and HSPC spatial gradient, (3) use of larger field-of-view marrow specimens through autopsy harvest, and (4) explicit consideration of active versus total bone marrow area.
Methods
Postmortem bone samples were collected during the autopsy of 9 recently deceased patients determined to be absent of marrow disease under a Health Insurance Portability and Accountability Act–compliant and University of Florida institutional review board–approved protocol that followed the Declaration of Helsinki provisions. Sections of bone were collected from the right iliac crest, L1 vertebrae, and 1 left rib, within 24 hours of death. Excised bone samples were placed in stock formaldehyde, rough sectioned, decalcified, and acid neutralized following the manufacturer's instructions (Formical-4 and Cal-Arrest; Decal Chemical Corp). The paraffin tissue blocks were faced and 4 sequential sections were collected from each specimen at a thickness of 5 μm.
Immunohistochemistry
Serial sections collected from decalcified, paraffin-embedded blocks were manually immunostained using mouse anti-CD34 (dilution 1:25, QBEND10; DAKO Cytomation) and rabbit anti-CD117 (dilution 1:300, c-Kit; DAKO Cytomation). Antigen retrieval was achieved using heat-induced epitope retrieval and treatment of slides with 10 mM Citra buffer (pH 6.0), after which slides were stained by the ABC-Elite method (Vector Labs) following the manufacturer's instructions. Positive signal was detected with diaminobenzidine (Vector Labs) and counterstained in hematoxylin QS (Vector Labs). Controls consisting of isotype- and concentration-matched immunoglobulin were included for each case. CD34 staining of endothelial cells served as an internal control. Cells were not considered CD117+ or CD34+ unless they exhibited visible membrane expression of the antigen, coupled with a small, primitive morphology consistent with a HSPC. In-plane arteries and blood vessel fragments were visually distinguishable from costained CD34+ cells by morphology, with the former appearing as tubular or elliptic structures depending on their orientation within the tissue section. Digital imaging was then performed as previously discussed by Watchman et al,8 and the distance of each BVF, or CD34+ or CD117+ HSPC to the nearest trabecular surface was measured and binned in 50-μm increments.
Results and discussion
Blood vessels
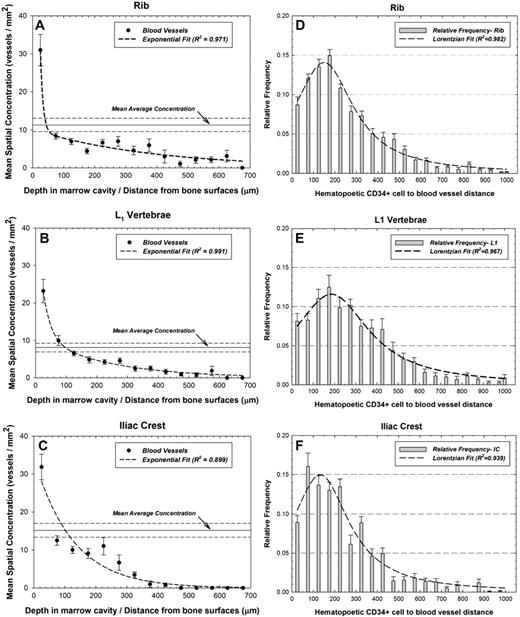
The ratio of total vessel count to total marrow area describes the average vessel concentration, and is represented by horizontal solid lines (dashed lines giving ± 1σ) in Figure 1A-C. Solid points represent experimental data binned in 50-μm increments. In each bone site, the greatest concentration of blood vessels is found between 0 and 50 μm from the trabecular surfaces, and the vessel concentration diminishes exponentially with increasing marrow depth. Figure 1D, 1E, and 1F display the relative frequencies of the distances between hematopoietic CD34+ cells and blood vessels in the rib, L1 vertebrae, and iliac crest, respectively. At all 3 bone sites, hematopoietic CD34+ cells were most likely to be found 100 to 200 μm from the nearest vessel structure. Similarly, approximately 9% of the cells were found within 50 μm of a vessel structure.
Spatial gradients of blood vessel fragments and cell-to-vessel distance frequencies. Mean areal concentrations of CD34+ vessels in active bone marrow as a function of distance into the marrow cavities of (A) ribs, (B) L1 vertebrae, and (C) iliac crest, and frequency distribution of distances separating pairs of hematopoietic cells and blood vessels within the marrow cavities of (D) ribs, (E) L1 vertebrae, and (F) iliac crest. Dashed line represents an exponential or Lorentzian fit to the data with R2 values shown. Also shown is the population mean average concentration (horizontal solid) ± 1σ (horizontal dashed).
Spatial gradients of blood vessel fragments and cell-to-vessel distance frequencies. Mean areal concentrations of CD34+ vessels in active bone marrow as a function of distance into the marrow cavities of (A) ribs, (B) L1 vertebrae, and (C) iliac crest, and frequency distribution of distances separating pairs of hematopoietic cells and blood vessels within the marrow cavities of (D) ribs, (E) L1 vertebrae, and (F) iliac crest. Dashed line represents an exponential or Lorentzian fit to the data with R2 values shown. Also shown is the population mean average concentration (horizontal solid) ± 1σ (horizontal dashed).
Hematopoietic CD34+ cells
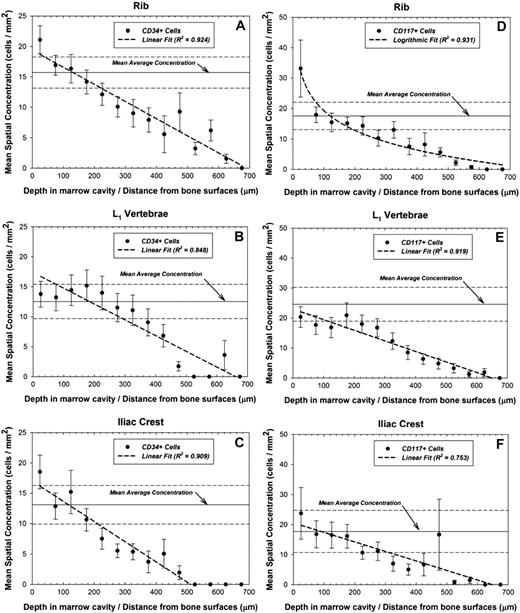
An identical analysis was performed to assess the spatial profile of hematopoietic CD34+ and CD117+ cells within the marrow cavities of human cancellous bone as shown in Figure 2A-F. In the rib, the HSPC concentration diminishes linearly by approximately 2% to 4% every 100 μm of marrow depth, with no cells found beyond 700 μm. The cellular concentration in the L1 vertebrae is essentially unchanged in the region between 0 and 250 μm, and statistically indistinguishable from the mean average concentration. However, at distances exceeding 250 μm, the HSPC concentration diminishes rapidly with no cells found beyond 700 μm. The CD34+ HSPC concentration in the iliac crest diminishes by approximately 2% to 5% for every 100 μm of marrow depth, and no cells are found beyond 550 μm.
Spatial gradients of CD34+ and CD117+ hematopoietic stem and progenitor cells in bone marrow. Mean areal concentrations of hematopoietic cells in active bone marrow as a function of distance into the marrow cavities of (A) ribs (CD34+), (B) L1 vertebrae (CD34+), (C) iliac crest (CD34+), (D) ribs (CD117+), (E) L1 vertebrae (CD117+), and (F) iliac crest (CD117+). Data points indicate specimen-averaged mean values binned every 50 μm, whereas the dashed line represents a logarithmic or linear fit to the data with R2 values shown. Also shown is the population mean average concentration (horizontal solid) ± 1σ (horizontal dashed).
Spatial gradients of CD34+ and CD117+ hematopoietic stem and progenitor cells in bone marrow. Mean areal concentrations of hematopoietic cells in active bone marrow as a function of distance into the marrow cavities of (A) ribs (CD34+), (B) L1 vertebrae (CD34+), (C) iliac crest (CD34+), (D) ribs (CD117+), (E) L1 vertebrae (CD117+), and (F) iliac crest (CD117+). Data points indicate specimen-averaged mean values binned every 50 μm, whereas the dashed line represents a logarithmic or linear fit to the data with R2 values shown. Also shown is the population mean average concentration (horizontal solid) ± 1σ (horizontal dashed).
Hematopoietic CD117+ cells
Figure 2D, E, and F plot the mean spatial concentration of CD117+ cells as a function of distance in the rib, vertebrae, and iliac crest, respectively. In the rib, a concentration of 33.1 (± 9.4) cells per square millimeter is found in the region between 0 and 50 μm, which is 88% higher than the mean average concentration. In the vertebrae, the mean average concentration is 24.6 (± 5.6) cells per square millimeter, higher than found in either the ribs or iliac crest. As with the CD34+ cell distribution in vertebrae, the CD117+ cell concentration is generally unchanged in the region between 0 and 250 μm. However, at distances greater than 250 μm, the CD117+ HSPC concentration rapidly diminishes in a linear fashion.
Bone and hematopoietic marrow is relatively resistant to autolysis compared with other body tissues, and antigenicity was well maintained in the postmortem bone specimens. Both CD34 and CD117 have been used in the literature as putative markers of stem cells. Although neither antibody is entirely specific for stem cells, the immunoreactivity of the cells coupled with their morphology review allow for distinct identification of stem cells. These differences are easily seen on immunostained materials. Due to the radiosensitivity and importance of HSPCs in marrow ablative therapies, the concentration and spatial location of blood vessels and the HSPC population in human cancellous bone has great clinical significance. In this study, a series of spatial profiles for hematopoietic CD117+ and CD34+ cells, as well as vascular fragments, are presented in the rib, vertebrae, and iliac crest. As can be seen in Figure 1, there was an approximately 2-fold increase in concentration of vascular fragments over the mean average concentration at distances less than 50 μm from the bone surface. We further note that bone-site differences in mean cellular concentration and their spatial gradients (Figure 2) are not sufficiently different to warrant changes in current clinical methods of reporting skeletal averaged radiation dose from either internal or external irradiation of the marrow tissues.
Conclusion
This study strongly supports the commonly accepted idea of the vascular niche being closely associated with the bone endosteum. Because approximately 50% of the hematopoietic CD34+ and CD117+ cells are found less than 200 μm from BVFs, this further implies a spatial niche that is concurrently occupied by the endosteum, BVFs, and HSPCs. Our current work was restricted in focus to specimens determined to be absent of bone marrow abnormalities. However, the inclusion of biopsy specimens commonly archived by medical institutions may allow investigation of various stem and progenitor cell spatial gradients within different bone marrow pathologies. Continued investigation of this topic may lead to improved understanding of the stem cell vascular niche and consequently new therapeutic approaches in the treatment of hematopoietic pathologies.9
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by grant RO1 CA96441 from the National Cancer Institute with the University of Florida.
National Institutes of Health
Authorship
Contribution: V.A.B. performed experiments and wrote the manuscript; C.J.W. assisted in data analysis; J.D.R. identified the patients; M.L.J. assisted in the experiments; A.D. developed software for image analysis and cellularity assessments; and W.E.B. designed and supervised the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wesley E. Bolch, 202 Nuclear Science Center, Advanced Laboratory for Radiation Dosimetry Studies (ALRADS), Department of Nuclear and Radiological Engineering, University of Florida, Gainesville, FL 32611-8300; e-mail: wbolch@ufl.edu.