Abstract
The ubiquitin-proteasome pathway, which degrades intracellular proteins, is involved in numerous cellular processes, including the supply of immunocompetent peptides to the antigen presenting machinery. Proteolysis by proteasomes is conducted by three β subunits, β1, β2, and β5, of the 20S proteasome. Recently, a novel β subunit expressed exclusively in cortical thymic epithelial cells was discovered in mice. This subunit, designated β5t, is a component of the thymoproteasome, a specialized type of proteasomes implicated in thymic positive selection. In this study, we show that, like its mouse counterpart, human β5t is expressed exclusively in the thymic cortex. Human β5t was expressed in approximately 80% of cortical thymic epithelial cells and some cortical dendritic cells. Human β5t was incorporated into proteasomes with two other catalytically active β subunits β1i and β2i, forming 20S proteasomes with subunit compositions characteristic of thymoproteasomes. The present study demonstrates, for the first time, the existence of thymoproteasomes in the human thymic cortex, indicating that thymoproteasome function is likely conserved between humans and mice.
Introduction
The proteasome constitutes the central proteolytic component of the highly conserved ubiquitin-proteasome system, which is required for the maintenance and regulation of basic cellular processes, including differentiation, proliferation, cell cycling, gene transcription, apoptosis, and the generation of antigenic peptides presented by major histocompatibility complex (MHC) class I molecules.1,2 Proteolysis is conducted in a core particle, known as the 20S proteasome, composed of two outer α-rings and two inner β-rings.3 In eukaryotes, the α- and β-rings are each made up of seven structurally similar α and β subunits. The proteolytic activity is exerted by three of the β subunits, β1, β2, and β5, which have caspase-like, trypsin-like, and chymotrypsin-like activities, respectively. In jawed vertebrates, interferon-γ (IFN-γ) produced in the initiation of immune responses induces the formation of a specialized type of 20S proteasomes in which β1, β2, and β5 subunits are replaced by three catalytically active, IFN-γ-inducible β subunits, β1i, β2i, and β5i, respectively. This type of proteasomes, known as immunoproteasomes, has been shown to efficiently produce antigenic peptides capable of binding to MHC class I molecules, thus facilitating elimination of virus-infected and tumor cells by cytotoxic T cells.4
Recently, a novel proteasome β subunit, which is closely related to β5 and β5i, was identified in humans and mice.5 In mice, this subunit, named β5t, is expressed exclusively in cortical thymic epithelial cells (cTECs) and is incorporated into 20S proteasomes together with β1i and β2i. This novel type of 20S proteasomes, named thymoproteasomes, appears to be involved in the development and repertoire formation of CD8+ T cells.5-7 β5 and β5i subunits have chymotrypsin-like proteolytic activity and contribute to the generation of peptides with hydrophobic C-terminal anchor residues.8 Peptides with these features generally bind to the clefts of MHC class I molecules with high affinity.9 In comparison to β5-containing standard proteasomes and β5i-containing immunoproteasomes, β5t-containing thymoproteasomes show only weak chymotrypsin-like activity but comparable caspase-like and trypsin-like activities.5 Thus, it has been speculated that thymoproteasomes facilitate production of peptides that bind to MHC class I molecules with only low affinity. Analysis of β5t-deficient mice showed that the generation of CD8+ single-positive thymocytes was severely impaired, suggesting that the thymoproteasome is involved in the development and presumably positive selection of T cells in the thymus.
In the present study, we show that, similar to mouse β5t, human β5t is expressed only in the thymic cortex and forms 20S proteasomes with subunit compositions characteristic of thymoproteasomes, indicating that thymoproteasome function is likely conserved between humans and mice. In addition, we show that some cortical dendritic cells (DCs) express β5t, suggesting their possible involvement in thymic selection.
Methods
Tissues
Human tissues were obtained from autopsy and surgical specimens with informed consent in accordance with the Declaration of Helsinki. All experiments were approved by the Medical Ethics Committee of Hokkaido University Graduate School of Medicine. Thymic tissues were fixed in 10% formalin or flash-frozen in liquid nitrogen and stored at −80°C.
Antibodies
Rabbit polyclonal antisera for human β5t were raised against the peptide encompassing resides 285 to 300 of human β5t. Anti-β5, -β5i, -β1, -β1i, -β2, -β2i, -β3, and -α6 antibodies (Abs) were described previously.5 Anti-keratin mAb (AE1/3) was purchased from Dako Japan (Tokyo, Japan). Biotinylated anti-CD11c mAb was from BD Biosciences (San Jose, CA). Biotinylated mAbs for CD209 and CD303 were from Miltenyi Biotec (Auburn, CA). Isotype-matched mouse IgG1, biotinylated mouse IgG1 (BD Biosciences), and pooled normal rabbit serum were used for negative controls. Alexa 488–conjugated goat anti–mouse IgG, Alexa 488-conjugated streptavidin, and Alexa 594–conjugated goat anti–rabbit IgG were from Invitrogen (Carlsbad, CA).
Immunofluorescence
Formalin-fixed tissue sections were deparaffinized in xylene and rehydrated in graded alcohols and distilled water. Slides were processed for antigen retrieval by a standard microwave heating technique. The slides were incubated in phosphate-buffered saline with 10% goat serum for 1 hour at room temperature, followed by overnight incubation with the primary Abs at 4°C. Sections were labeled with secondary Abs or Alexa 488–conjugated streptavidin and tested by fluorescence microscope. Cryostat sections were acetone-fixed and stained with Abs in the same manner. Slides were viewed with a Nikon Eclipse E600 microscope (Nikon, Tokyo, Japan). Images were acquired using an Olympus DP70 camera with its own Olympus DP controller software (Olympus, Tokyo, Japan).
Reverse transcription–polymerase chain reaction
Total RNAs were isolated from tissues using RNeasy Spin columns (QIAGEN, Valencia, CA), treated with DNase I, and converted to cDNA with the Superscript III kit (Invitrogen). cDNAs were subjected to polymerase chain reaction (PCR) using the following oligonucleotide primers for human β5t, β1i, β2i, and GAPDH (sense and antisense, respectively): β5t, 5′-GCTTCCGTCATGGAGTCATT-3′ and 5′-AGAGGTGGTACCCAGGAGGT-3′; β1i, 5′-ATGCTGACTCGACAGCCTTT-3′ and 5′-GCAATAGCGTCTGTGGTGAA-3′; β2i, 5′-GTGCTAGAAGACCGGTTCCA-3′ and 5′-ATCACACATGCGTCCACATT-3′; and GAPDH, 5′-AGCGAGATCCCTCCAAAATC-3′ and 5′-GGCAGAGATGATGACCCTTT-3′. Cycling conditions were 1 cycle at 95°C for 2 minutes, followed by 35 cycles of denaturation at 95°C for 20 seconds, and annealing/extension at 57°C for 1 minute.
Immunoprecipitation and Western blot analysis
Thymic tissues were lysed in a buffer containing 150 mM NaCl, 20 mM Tris-HCl (pH 7.5), 0.2% NP-40, and 1 mM dithiothreitol (DTT), and centrifuged at 15 000g for 10 minutes. The supernatants (10 μg aliquot of total proteins) were subjected to sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis and blotted to nitrocellulose membranes. The blots were probed with Abs and were reacted with horseradish peroxidase-conjugated anti–rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for immunodetection. The immunocomplexes were visualized by enhanced chemiluminescence (GE Healthcare, Little Chalfont, United Kingdom) and were analyzed by Image Gauge software (Fujifilm, Tokyo, Japan). For immunoprecipitation, tissue lysates (100 μg of protein) were incubated in a total volume of 150 μL of 25 mM Tris-HCl buffer (pH 7.5) containing 1 mM DTT at 4°C for 2 hours with anti-α6 or anti-β5t Ab bound to protein A Sepharose (GE Healthcare). The beads were washed and boiled in SDS sample buffer. After centrifugation, supernatants were used for Western blot analysis. Each lane was loaded with 15% of the total amount of proteasomes precipitated with the anti-α6 or anti-β5t Ab.
Results
Human β5t is expressed specifically in the thymus
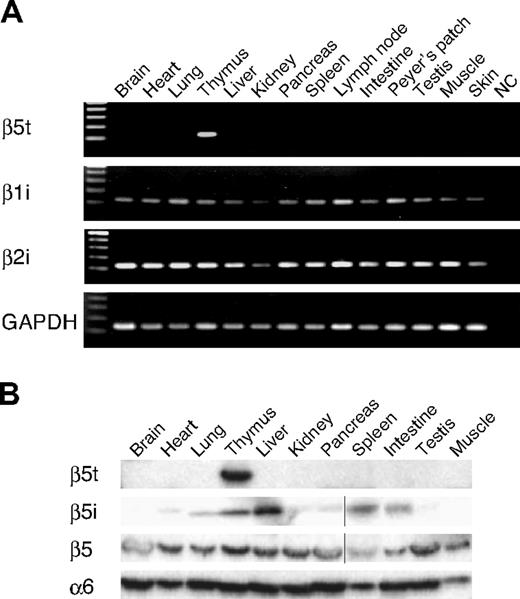
To examine the expression profiles of β5t, we first performed reverse transcription (RT)–PCR and Western blot analysis using representative human tissues. As shown in Figure 1A, β5t mRNA was detected only in the thymus among the tissues examined. Thymus-specific expression of the β5t gene was confirmed by analysis of expressed sequence tag sequences deposited in the GenBank of 45 human organs/tissues (see expression profiles for UniGene, http://www.ncbi.nlm.nih.gov/unigene/, Hs. 508918). Because mouse β5t functions together with IFN-γ–inducible β subunits, β1i and β2i, we also examined the expression of β1i and β2i mRNAs in human tissues. β1i and β2i mRNAs were detected in all of the tissues tested, including thymus. Immunoblot analysis using the antisera for human β5t detected a band of expected size (∼ 32 kDa) only in the thymus, thus confirming thymus-specific expression of β5t and the specificity of the antisera (Figure 1B). β5 was expressed in all the tissues tested, whereas β5i was detected mainly in lymphoid tissues, liver, and lung. The distribution of β5, β5i, and β5t subunits in human tissues was quite similar to that previously observed in mouse tissues.5
Human β5t is expressed specifically in the thymus. (A) RT-PCR analysis of β5t, β1i, and β2i expression in human tissues. NC indicates negative control. (B) Immunoblot analysis of human tissues with indicated Abs. Vertical lines have been inserted to indicate a repositioned gel lane.
Human β5t is expressed specifically in the thymus. (A) RT-PCR analysis of β5t, β1i, and β2i expression in human tissues. NC indicates negative control. (B) Immunoblot analysis of human tissues with indicated Abs. Vertical lines have been inserted to indicate a repositioned gel lane.
β5t is expressed at high levels in the majority of cTECs
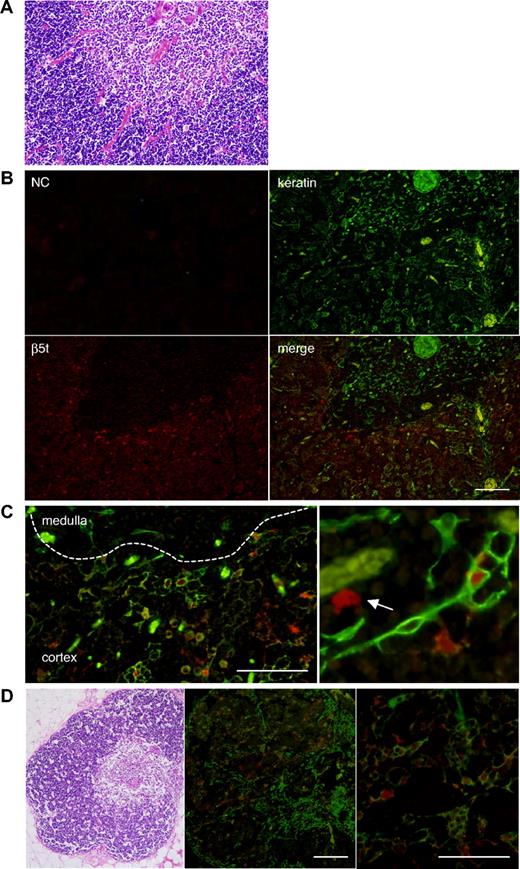
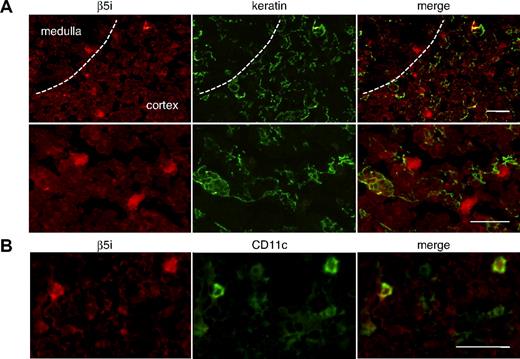
To identify cells that express β5t in the thymus, neonatal thymic tissues were stained with anti–human β5t and anti–keratin Abs (Figure 2). β5t-expressing cells were present in the cortex but not in the medulla. The majority of cells strongly stained with anti-β5t Ab were stained with the Ab for keratin, indicating that β5t is expressed primarily in cTECs (Figure 2B). Approximately 80% of cTECs were stained with the Ab for β5t. Interestingly, a small number of β5t-expressing cells were not stained with the Ab for keratin, suggesting that cells other than cTECs also express β5t (Figure 2C).
Human β5t is expressed exclusively in the thymic cortex. (A) Hematoxylin and eosin (HE) staining of neonatal human thymic tissues. (B,C) Immunofluorescence staining of neonatal human thymic tissues. The medulla is identified by the presence of Hassall corpuscles and lower cell density than in the cortex. β5t-expressing cells are located only in the cortex, and most of the β5t-expressing cells in the cortex are keratin-positive cTECs. Magnified images of immunostaining (C) reveal that β5t is expressed in cTECs, but some β5t-expressing cells are not stained with the Ab for keratins (arrow). The staining patterns shown were representative of three neonatal thymic tissues. Negative control (NC) was stained with normal rabbit serum and mouse IgG1. (D) HE and immunofluorescence staining of adult human thymus. Expression of β5t is maintained in cTECs. The staining patterns shown were representative of five adult thymic tissues. Red represents β5t; green, keratin. Bar indicates 100 μM.
Human β5t is expressed exclusively in the thymic cortex. (A) Hematoxylin and eosin (HE) staining of neonatal human thymic tissues. (B,C) Immunofluorescence staining of neonatal human thymic tissues. The medulla is identified by the presence of Hassall corpuscles and lower cell density than in the cortex. β5t-expressing cells are located only in the cortex, and most of the β5t-expressing cells in the cortex are keratin-positive cTECs. Magnified images of immunostaining (C) reveal that β5t is expressed in cTECs, but some β5t-expressing cells are not stained with the Ab for keratins (arrow). The staining patterns shown were representative of three neonatal thymic tissues. Negative control (NC) was stained with normal rabbit serum and mouse IgG1. (D) HE and immunofluorescence staining of adult human thymus. Expression of β5t is maintained in cTECs. The staining patterns shown were representative of five adult thymic tissues. Red represents β5t; green, keratin. Bar indicates 100 μM.
The thymus undergoes a slow physiologic process of involution with age. Underlying causes of thymic involution are poorly understood, but it has been speculated that involution occurs as a result of the degeneration of the stromal thymic network that provides survival and differentiation factors for developing T cells, or of the insufficiency of stromal and hematopoietic progenitor cells.10,11 We detected β5t-expressing cTECs in adult thymic tissues that underwent spontaneous involution, indicating that expression of β5t persists to adulthood (Figure 2D). This result may consistent with the idea that the thymus supports the development of T cells throughout life.12,13
Some DCs in the thymic cortex express β5t
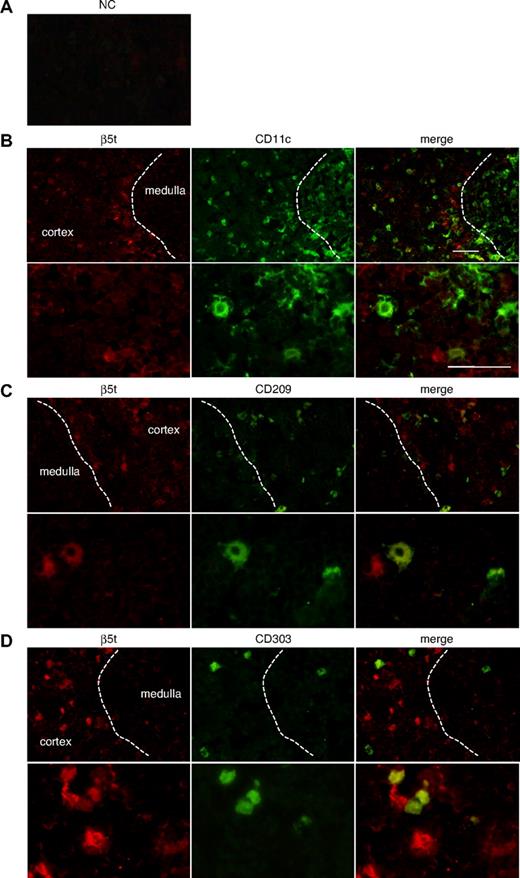
To establish the identity of β5t-positive cells that do not express keratins, we performed immunostaining analysis using various markers for DCs, such as CD11c, CD209 (DC-SIGN), and CD303 (BDCA-2) (Figure 3). DCs were chosen as candidates for β5t-positive cells because they are known to interact closely with developing thymocytes. CD11c-positive cells were detected predominantly in the medulla but were also located in the cortex, whereas CD209-positive cells were distributed exclusively in the cortex. β5t was weakly expressed in a small number of CD11c- or CD209-positive cells in the cortex (Figure 3B,C). Cells expressing CD303, a marker for plasmacytoid DCs, were scattered throughout the cortex and medulla, and β5t was expressed in some CD303-positive cells in the cortex (Figure 3D). Thus, β5t is expressed in some cortical DCs, including myeloid DCs, DC-SIGN-positive DCs, and plasmacytoid DCs, indicating that expression of β5t is not restricted to a specific population of DCs in the cortex.
β5t is expressed in some thymic DCs. Neonatal thymic sections were immunostained for β5t and markers for DCs: (A) NC, (B) CD11c, (C) CD209, and (D) CD303. NC was stained with normal rabbit serum and biotinylated mouse IgG1. The lower half of each panel shows a magnified image of the cortical area. Bar represents 50 μM.
β5t is expressed in some thymic DCs. Neonatal thymic sections were immunostained for β5t and markers for DCs: (A) NC, (B) CD11c, (C) CD209, and (D) CD303. NC was stained with normal rabbit serum and biotinylated mouse IgG1. The lower half of each panel shows a magnified image of the cortical area. Bar represents 50 μM.
β5t is incorporated into 20S proteasomes together with β1i and β2i in cTECs
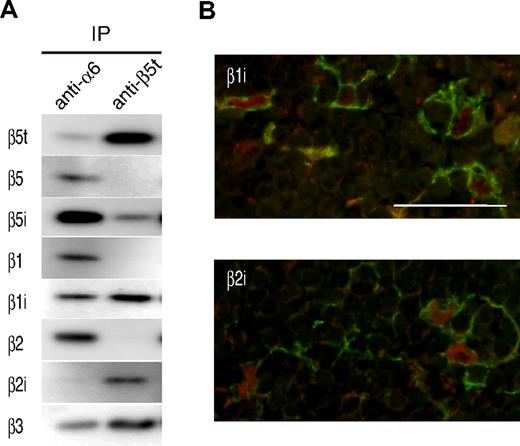
We next examined whether human β5t could be incorporated into 20S proteasomes in a manner similar to mouse β5t. The lysate obtained from neonatal thymic tissues was immunoprecipitated with Abs for α6 or β5t. All three β5-type subunits, β5, β5i, and β5t, were detected in thymic 20S proteasomes, of which β5i was most abundant (Figure 4A left panel). By contrast, β5 was virtually absent, and β5i was only weakly detected in β5t-containing proteasomes, indicating that β5t was incorporated into 20S proteasomes in place of β5 or β5i (Figure 4A right panel). In addition, β5t-containing proteasomes contained IFN-γ–inducible subunits β1i and β2i, but virtually no β1 or β2 subunits. Because β1i and β2i were preferentially detected in cTECs by immunostaining analysis (Figure 4B), combined data indicate that β5t is incorporated into 20S proteasomes together with β1i and β2i in cTECs. These results indicate that human β5t forms a unique type of proteasomes with subunit compositions identical to those of mouse thymoproteasomes.
β5t is incorporated into 20S proteasomes with β1i and β2i in the human thymus. (A) Extracts of human neonatal thymi were immunoprecipitated with anti-α6 or anti-β5t Ab, followed by immunoblotting with the indicated Abs. IP indicates immunoprecipitation. (B) Immunofluorescence staining of the neonatal thymus using anti-β1i or anti-β2i Ab. Each panel shows an image of the cortical area. β1i and β2i (in red) are expressed in keratin-expressing cTECs (in green). Bar represents 50 μM.
β5t is incorporated into 20S proteasomes with β1i and β2i in the human thymus. (A) Extracts of human neonatal thymi were immunoprecipitated with anti-α6 or anti-β5t Ab, followed by immunoblotting with the indicated Abs. IP indicates immunoprecipitation. (B) Immunofluorescence staining of the neonatal thymus using anti-β1i or anti-β2i Ab. Each panel shows an image of the cortical area. β1i and β2i (in red) are expressed in keratin-expressing cTECs (in green). Bar represents 50 μM.
β5i is expressed at high levels in cortical DCs
It has been demonstrated that thymic stromal cells mediating negative selection, such as medullary thymic epithelial cells (mTECs) and DCs, express immunoproteasomes in the mouse thymus.14 In addition, immunoproteasomes are known to affect CD8+ T-cell repertoire selection.15 To identify major cell populations that express β5i in the human thymus, we stained neonatal thymic tissues with anti-β5i, and anti-keratin or CD11c Abs (Figure 5). Consistent with the result of immunoprecipitation with anti-α6 (Figure 4A), β5i-expressing cells were distributed abundantly in both cortex and medulla (Figure 5A). In the cortex, cells expressing β5i at high levels were not stained with Ab for keratin but stained with Ab for CD11c, indicating that β5i is predominantly expressed in cortical DCs and, unlike β1i and β2i, is barely or not at all expressed in cTECs (Figure 5B). DCs in the medulla were also intensely stained with Ab for β5i (data not shown).
β5i is widely expressed in the human thymus. (A) β5i-expressing cells are distributed in both cortex and medulla and are not stained with Ab for keratin. The lower panel shows a magnified image of the cortical area. (B) Cells expressing β5i at high levels in the cortex are stained with Ab for CD11c. Thus, cortical DCs express β5i at high levels. Bar represents 50 μM.
β5i is widely expressed in the human thymus. (A) β5i-expressing cells are distributed in both cortex and medulla and are not stained with Ab for keratin. The lower panel shows a magnified image of the cortical area. (B) Cells expressing β5i at high levels in the cortex are stained with Ab for CD11c. Thus, cortical DCs express β5i at high levels. Bar represents 50 μM.
Discussion
During the development of T cells, only a small proportion of thymocytes is allowed to survive and leave the thymus as mature T cells. These selection events in thymocyte development require the interaction of the T-cell receptor with self-peptide/MHC complexes, and both cTECs and mTECs have an important role in displaying self-peptides to thymocytes. In this study, we have shown that human β5t is expressed exclusively in the thymic cortex. Although it is expressed predominantly in cTECs, some cortical DCs also express β5t. Human β5t was incorporated into 20S proteasomes with two other catalytic subunits, β1i and β2i, forming proteasomes with subunit compositions identical to those of mouse thymoproteasomes. Previous analysis in mice showed that thymoproteasomes are expressed exclusively in cTECs and play a critical role in generating MHC class I-restricted CD8+ T-cell repertoire.5 Furthermore, the cleavage specificity of thymoproteasomes predicted preferential generation of low-affinity MHC class I ligands, suggesting their role in positive selection of T cells.5-7 Our current work, which demonstrated thymoproteasomes in human cTECs, indicates that they may also be involved in positive selection of human T cells.
In the human thymus, several discrete populations of DCs, such as immature myeloid, mature myeloid, and plasmacytoid DCs, have been identified using combinations of phenotypic markers.16,17 It is widely accepted that thymic DCs are largely distributed in the medulla and play a crucial role in the process of negative selection.18 However, recent evidence indicates that they are also present in the cortex and can mediate positive selection.19 For example, CD209-positive cells located in the human thymic cortex exhibit features of both immature DCs and macrophages and appear to have unique functions in thymic selection and removal of apoptotic thymocytes.20 In line with this observation, we observed that some cortical thymic DCs express β5t (Figure 3). Because DCs with various phenotypic markers (CD11c, CD209, or CD303) express β5t, expression of β5t is probably not restricted to a specific population of DCs. Thus, distinct populations of thymic cortical DCs appear to express β5t under appropriate conditions. In addition, it is notable that human proteasomes immunoprecipitated with anti-β5t contained a small amount of β5i (Figure 4A right panel). Similar experiments in mice failed to detect β5 or β5i.5 Coprecipitation of human β5t and β5i presumably occurs because human thymus contains a small amount of an unusual form of 20S proteasomes formed by dimerization of half-proteasomes, each containing β5t and β5i. Because cortical DCs have the potential to express β5t, some of them may coexpress β5i and β5t, leading to the formation of 20S proteasomes, containing β5i in one half-proteasome and β5t in another. DCs derived human peripheral blood monocytes are known to express both proteasomes and immunoproteasomes.21 In addition, it has been demonstrated in mammalian cells that the replacement of the constitutive subunits (β1, β2, and β5) by IFN-γ-inducible subunits (β1i, β2i, and β5i) results in the formation of asymmetric proteasomes with one half-proteasome containing β5 and another containing β5i, exhibiting different proteolytic activities.22 In an analogous manner, some thymic cortical DCs may express asymmetric proteasome particles containing both β5t and β5i subunits. Further research is required to understand the biologic significance of asymmetric proteasomes. Currently, the composition of proteasomes in thymic DCs is not well understood. It is probable that, besides asymmetric proteasomes, cortical DCs express thymoproteasomes and immunoproteasomes. However, biochemical studies using isolated cortical thymic DCs are required to address whether this is indeed the case and to determine the relative abundance of the distinct types of proteasomes.
The mechanism regulating the expression of β5t in the thymus is still unknown. Interestingly, the majority of cTECs express β5t but not β5i, suggesting that cTECs might have the transcriptional machinery that specifically up- and down-regulates the expression of β5t and β5i, respectively. Alternatively, the apparent absence of β5i expression in cTECs may occur posttranslationally through preferential incorporation of β5t into 20S proteasomes and subsequent degradation of unincorporated β5i. To elucidate unique proteasomal processing of self-Ags in the thymus, it would be important to clarify the mechanisms regulating the expression of β5t and β5i in cTECs and other thymic stromal cells.
In conclusion, our study demonstrates, for the first time, the existence of thymoproteasomes in the human thymic cortex and suggests that thymoproteasomes probably have an important role in positive selection of human T cells as well. Future studies should address whether abnormalities of β5t expression are involved in the development of human diseases. Alteration of β5t expression might result in aberrant T-cell development, leading to immune dysfunctions, such as autoimmune diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Hitoshi Ikeda for the supply of thymic tissues and Chisato Sudo and Kenichi Nakase for technical support.
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Authorship
Contribution: U.T. designed the work, performed experiments, and wrote the manuscript; A.I. designed the research and analyzed the data; S.M. provided Abs and performed immunoprecipitation; Y.M., S.S., S.T., T.K., and J.O. performed molecular analysis and Western blot analysis; T.B. contributed to immunofluorescence analysis; S.I., K.F., and N.O. contributed to histologic analysis; K.T. supervised the research design; and M.K. supervised the entire study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Utano Tomaru, Kita-15, Nishi-7, Kita-ku, Sapporo 060-8638, Japan; e-mail: tomaruu@med.hokudai.ac.jp.