In this issue of Blood, Zapata and colleagues investigate a novel transgenic mouse model overexpressing TRAF3 in B cells and surprisingly find plasmacytosis and hypergammaglobulinemia, even though TRAF3 is reported to be a tumor suppressor gene in MM.
Homozygous inactivation of tumor necrosis factor (TNF)–receptor-associated factor 3 (TRAF3) was recently reported in 13% of multiple myeloma (MM) patients.1,2 It has been found to be a negative regulator of NIK, which, as expected, is elevated in MM with TRAF3 inactivation. NIK is a key enzyme regulating processing of NFKB2 p100 to p52 that dimerizes with relB and results in transcriptional activation of NFkB targets. Highlighting the importance of NIK stabilization, additional mutations with similar functional consequences occur in MM, including biallelic deletions encompassing both cIAP1 and cIAP2, as well as biallelic deletions of TRAF2, all contributing together with TRAF3 to form a NIK negative regulatory complex.3 Based on constitutive p100 processing, and the presence of nuclear p52-relB complexes in TRAF2 or TRAF3 deficient B cells, they have been described as “inhibitors” of the alternative NF-kB pathway.4 Whereas tumors with dysregulated overexpression of TRAF3 have not been reported, amplification and overexpression of cIAP1/2 occurs in various tumors and has been shown to be critical in liver cancer.5
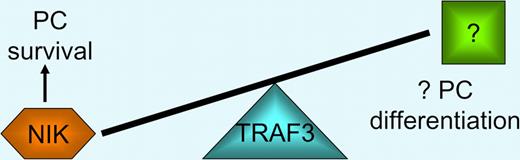
TRAF3 is a critical regulator of plasma cell homeostasis. TRAF3 is an adaptor that recruits a TRAF2, cIAP1, and cIAP2 E3 ubiquitin ligase complex to NIK, leading to rapid turnover in resting B cells. Low levels of TRAF3 lead to NIK stabilization, activation of the alternative NF-kB pathway, and plasma cell survival. High levels of TRAF3 in transgenic B cells results in reduced B-cell numbers, but plasmacytosis by an unresolved mechanism, perhaps related to increased plasma cell differentiation.
TRAF3 is a critical regulator of plasma cell homeostasis. TRAF3 is an adaptor that recruits a TRAF2, cIAP1, and cIAP2 E3 ubiquitin ligase complex to NIK, leading to rapid turnover in resting B cells. Low levels of TRAF3 lead to NIK stabilization, activation of the alternative NF-kB pathway, and plasma cell survival. High levels of TRAF3 in transgenic B cells results in reduced B-cell numbers, but plasmacytosis by an unresolved mechanism, perhaps related to increased plasma cell differentiation.
Consistently, mice with conditional inactivation of TRAF3 in B cells develop splenomegaly and lymphadenopathy due to B-cell expansion, and hypergammaglobulinemia with increased plasma cell numbers.6 On the other hand, the TRAF3 transgenic mice described in the current report have lower levels of NIK with splenic hypotrophy and reduced numbers of B cells.7 The unexpected finding, however, is that elevated TRAF3 in these mice also results in hypergammaglobulinemia and plasmacytosis. Zapata et al have shown that this phenotype is not due to increased B-cell proliferation, but suggest it may be the result of heightened Toll-like receptor (TLR)–mediated B-cell differentiation. Interestingly, the mice develop a high incidence of solid tumors (67%) with age, primarily squamous cell carcinoma of the tongue, which the authors suggest may result from the chronic inflammation present in these mice. As noted, this represents the first model of spontaneous carcinogenesis initiated by B-cell mediated chronic inflammation. A potential limitation of these studies is that only a single transgenic founder line was examined in detail, and the phenotype may potentially relate to the site of transgene insertion in this founder.
Additional studies of these fascinating mice will be required to better understand the mechanisms underlying the many surprising observations these mice afford.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■