In this issue of Blood, Kirshner and colleagues describe the development of an exciting 3-dimensional model system that appears to support in vitro expansion of primary myeloma cells and provides a physiologically relevant platform on which to test the therapeutic efficacy of antimyeloma drugs.
Currently, the arsenal of experimental tools available to study the biology and drug susceptibility of primary human myeloma cells is significantly restricted by the lack of in vitro model system(s) that permits reproducible growth of primary myeloma cells, including the putative cancer stem cell compartment. Investigators in this field have long struggled with this limitation and have concluded that this experimental hurdle largely reflected the ill-defined, yet critical, dependence of primary myeloma cells on signals uniquely provided by the bone marrow microenvironment and the failure of various in vitro systems to provide this support. Indeed, it is not without coincidence that the vast majority of human myeloma cell lines established to date derive from patients who have progressed to extramedullary disease,1 implying that success in this arena is tied to the relative emancipation of at least a subset of primary myeloma cells from this environment. Moreover, the critical role of the bone marrow microenvironment is also reinforced by the success that Yaccoby et al2 and Yata et al 3 have enjoyed with their in vivo model of primary myeloma cell growth. In these reports, the ability to achieve reproducible growth of primary myeloma cells was critically dependent upon injection of tumor cells into either human fetal2 or rabbit fetal bone xenografts3 established in immunocompromised mice. Despite the success of both models, there are significant technical challenges, and access to xenograft material is limited particularly as it concerns human fetal bone. Regarding in vitro models, Matsui et al4 used a methylcellulose clonogenic assay to demonstrate growth of putative CD138-negative myeloma cancer stem cells, and in later studies this system was used to analyze susceptibility of these cells to various therapeutic agents.5 Although studies of this nature do allow an evaluation of the therapeutic sensitivity of the myeloma cells that survive or proliferate under these conditions, they do not address the possibility that signals from the microenvironment may lend a protective hand to the bone marrow–resident tumor cells. This concept is well recognized in many types of human malignancies, including myeloma.6
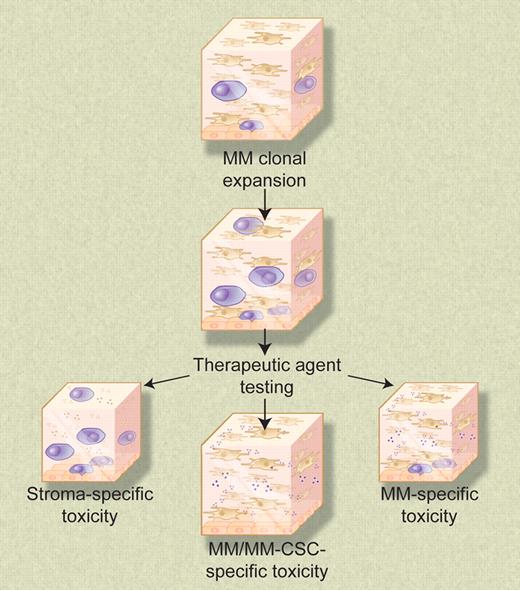
The 3-dimensional reconstructed bone marrow model system permits expansion of numerous cell types, including clonal myeloma cells. Nonproliferating myeloma cells concentrate at the reconstructed endosteal-marrow junction and may represent drug-resistant myeloma cancer stem cells. This biologically relevant model system provides a means to test the sensitivities of all cellular components of the myeloma clone, as well as test for undesirable toxic side effects on other cell types naturally present in the bone marrow microenvironment. Illustration by Debra T. Dartez.
The 3-dimensional reconstructed bone marrow model system permits expansion of numerous cell types, including clonal myeloma cells. Nonproliferating myeloma cells concentrate at the reconstructed endosteal-marrow junction and may represent drug-resistant myeloma cancer stem cells. This biologically relevant model system provides a means to test the sensitivities of all cellular components of the myeloma clone, as well as test for undesirable toxic side effects on other cell types naturally present in the bone marrow microenvironment. Illustration by Debra T. Dartez.
In the current study, Kirshner and colleagues set upon the ambitious task of developing a dual purpose in vitro model system that would: (1) support reproducible primary myeloma cell growth, including the putative myeloma stem cell; and (2) permit assessment of the effectiveness of antimyeloma therapeutic agents in a setting that mimics the natural microenvironment of bone marrow–resident malignant plasma cells. The introductory remarks stated a priori predict that success at achieving both aspects of the desired model would pivot around success in construction of the bone marrow microenvironment. To favor success, the authors focused on developing a 3-dimensional tissue culture model that would permit in vitro reconstruction of the human bone marrow microenvironment. This 3-dimensional system consisted of 3 essential elements. First, the authors coated tissue culture wells with a collagen I/fibronectin mixture to reconstruct the endosteum. The next layer in the system was reconstructed bone marrow, which was created by mixing bone marrow mononuclear cells with an extracellular matrix mixture of Matrigel (BD Biosciences, Mississauga, ON) and fibronectin. The third layer consisted of growth medium, of which a critical factor was plasma from blood samples obtained from myeloma patients. Curiously, plasma from healthy donors did not support myeloma-cell growth. In this model, stromal cells migrate to the simulated endosteum and cover the bottom of the well. Other cell types remain distributed throughout the 3-dimensional matrix. The authors' data suggest that this system indeed recapitulates the in vivo bone marrow microenvironment, including physical stratification between the endosteum and central marrow and differential localization of various members of the myeloma clone within the 3-dimensional model. This latter feature is attractive because it potentially allows an assessment of the therapeutic agent sensitivity of both more differentiated central marrow-resident myeloma cells and the putative less differentiated and more dormant endosteum-associated myeloma cancer stem cells.
It is appealing to propose that the experimental system described here, which appears to support either survival or the expansion of all members of the myeloma clone, will be of broad use to investigators studying myeloma-cell biology and/or antimyeloma chemotherapeutic or biologicallybased agents. This 3-dimensional reconstituted bone marrow system may likewise be beneficial to investigators studying other human B-lineage malignancies. From a practical standpoint, however, given the intricacies of this model system, assessment of its true impact on the field of myeloma research will await successful implementation of this methodology in other laboratories.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■