To the editor:
In 1998 a female patient received an allogeneic stem cell (SC) transplant from her sister 9 months after she was diagnosed with a high risk myelodysplastic syndrome (RAEB2) and was treated with chemotherapy aiming to achieve complete remission. A myeloablative conditioning regime was followed by infusion of peripheral blood stem cells (PBSC) from the donor. Two years later, a second myeloablative SC transplantation (SCT) with PBSC from the same donor was performed because of relapse. Seven years after the second SCT, the patient continues to be in complete remission with persistent complete donor chimerism.
The sister of the above-mentioned patient was found to be the only HLA-matched sibling donor. Blood examination 5 months before the first PBSC collection revealed an unexplained leukocytosis (19 × 109/L) and thrombocytosis (750 × 109/L). Both resolved spontaneously after 2 months. Three months before the first PBSC collection the donor also presented with a portal venous thrombosis (PVT). Additional analyses, including coagulation studies, bone marrow biopsy, and human androgen receptor (HUMARA) assay revealed no evidence of an underlying thrombophilia or clonal myeloproliferative disorder (MPD). Both donor and recipient have normal blood counts after more than 7 years' follow-up and have not developed overt MPD.
After the report of JAK2V617F mutations in the majority of Philadelphia− MPDs,1-3 donor and recipient samples were analyzed (method kindly provided by H. El Housni, Department of Medical Genetics, Free University of Brussels, Brussels, Belgium).4 Informed consent was obtained in accordance with the Declaration of Helsinki. The assay showed no background amplification on DNA of healthy blood donors. Moreover, JAK2V617F positivity was confirmed by M. Girardot (laboratory of S.N.C.).5
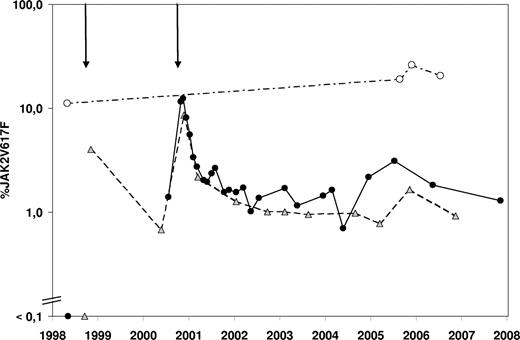
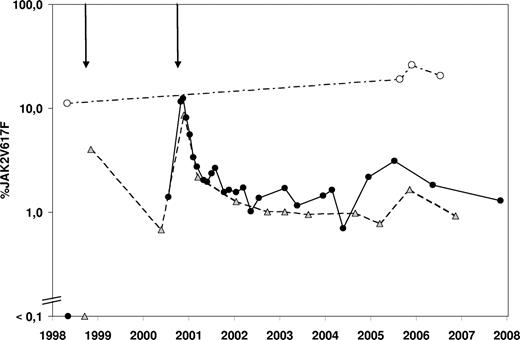
The donor was found to be JAK2V617F+ in all samples tested (Figure 1), which may explain the PVT and the episode of transient leukocytosis and thrombocytosis observed before the first PBSC collection.6 In the recipient, the mutation was confirmed to be absent before the first transplantation. Immediately after the second transplantation a steep increase in the JAK2V617F mutational burden was seen, which decreased significantly over the course of 1 year to approximately 1% and still continues to be detectable at this level. At least 2 hypotheses may explain this observation. First, the heterogeneity of the transplanted stem cell pool might be different with respect to the original donor stem cell pool. Second, the transplanted JAK2V617F+ hematopoietic stem cells (HSCs) might have exhibited an intrinsic homing or proliferative disadvantage compared with the JAK2 wild type HSCs, possibly due to a different bone marrow microenvironment. Interestingly, specific genetic variations were recently found to be associated with the different clinical phenotypes of JAK2V617F+ MPDs.7 These or other genetic loci could be responsible for a different composition of the bone marrow microenvironment.
Evolution over time of the JAK2V617F mutational burden. The percentage JAK2V617F value represents the ratio of JAK2V617F mutated DNA to all JAK2 DNA present in a sample as measured by quantitative polymerase chain reaction. The 2 arrows above the graph represent the first and second allogeneic transplantations. Donor samples (○) are connected with a dashed-pointed line, recipient peripheral blood samples (■) are connected with a full line, and recipient bone marrow samples ( ) are connected by a dashed line.
) are connected by a dashed line.
Evolution over time of the JAK2V617F mutational burden. The percentage JAK2V617F value represents the ratio of JAK2V617F mutated DNA to all JAK2 DNA present in a sample as measured by quantitative polymerase chain reaction. The 2 arrows above the graph represent the first and second allogeneic transplantations. Donor samples (○) are connected with a dashed-pointed line, recipient peripheral blood samples (■) are connected with a full line, and recipient bone marrow samples ( ) are connected by a dashed line.
) are connected by a dashed line.
In conclusion, the fact that 7 years after transplantation a low number of hematopoietic cells still harbors the JAK2V617F mutation definitively confirms that progenitor cells with long-term repopulating capacities are targets for this mutation. This case also strongly suggests that the JAK2V617F mutation does not necessarily result in a proliferative advantage at the stem cell level and alone might not be sufficient for development of an MPD phenotype.
Authorship
Nadine Mestdagh and Astrid Denys are acknowledged for technical assistance. We thank Michael Girardot for confirmatory tests on JAK2V617F positivity.
This study was supported by Wetenschappelijk Fonds Hematologie v.z.w. Generous support to S.N.C. and L.K. from the Salus Sanguinis Foundation, the Action de Recherche Concertée MEXP31C of the Université Catholique de Louvain, the Interuniversity Attraction Poles program of the Belgian Federal Government, the Fonds de la Recherche Scientifique (FNRS) Belgium and the Atlantic Philantropies/Ludwig Institute research program is acknowledged.
Contribution: K.V.P., F.N., and J.B. performed the JAK2V617F analyses and wrote the manuscript; D.S. provided all samples and clinical data; L.K. and S.N.C. provided interesting hypotheses and confirmed JAK2V617F positivity; all authors were involved in critical reviewing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johan Billiet, Laboratory of Hematology, AZ Sint-Jan AV, Ruddershove 10, B-8000 Brugge, Belgium; e-mail: Johan.Billiet@azbrugge.be.


 ) are connected by a dashed line.
) are connected by a dashed line.