Abstract
Natural killer (NK)–cell alloreactivity is exploited in bone marrow transplantation to improve clinical outcome. Likewise, in solid organ transplantation, it has been recently shown that recipient NK cells may limit alloreactive T-cell responses through their capacity to prevent the persistence of graft-derived allogeneic dendritic cells (DCs). In a model of CD4+ T cell–mediated allogeneic skin graft rejection, we show that the absence of host NK-cell alloreactivity was characterized by enhanced expansion of alloreactive effector T lymphocytes, including Th2 cells, and massive eosinophilic infiltrates in the rejected tissues. In CD8+ T cell–deficient C57BL/6 (H-2b) recipients injected with allogeneic BALB/c (H-2d) DCs, we demonstrated that NK cells expressing the H-2Dd-specific Ly49D activating receptor were implicated in the regulation of alloreactive CD4+ T-cell responses. Moreover, we showed that Ly49D+ CD127− NK cells were recruited within DC draining lymph nodes and rapidly eliminated allogeneic H-2d DCs through the perforin pathway. In normal mice, we further demonstrated that NK cells by quickly eliminating allogeneic DCs strongly inhibited alloreactive CD8+ T-cell responses. Thus, NK cells act as early regulators of alloreactive T-cell priming in allotransplantation through their capacity to kill allogeneic DCs in draining lymph nodes.
Introduction
Natural killer (NK) cells are a major component of the innate immune system and are capable of killing target cells without prior immunization.1 Activation of NK cells results from the balance between the engagement of complex families of inhibitory and activatory receptors specific for major histocompatibility complex (MHC) I molecules and various others ligands.2,3 Inhibitory receptors encompass several families of cell surface molecules, such as Ly49 and CD94/NKG2 receptors in mouse, that recognize self-MHC class Ia molecules and transmit intracellular signals preventing NK-cell activation and target cell lysis.2,3 Activating receptors recognize MHC class I-like molecules and other undefined ligands and trigger lysis and cytokine production.3 Engagement of an excess of inhibitory receptors aborts the activating signaling cascades. Consequently, in allotransplantation settings, interaction with fully mismatched allogeneic cells, lacking self MHC I molecules, leads to unopposed activating signals and NK-cell activation characterized by target cell killing and cytokine release.4
Their ability to distinguish allogeneic cells from self and their potent cytolytic effector function make NK cells relevant to allogeneic bone marrow transplantation.5 However, their role in the rejection of organ grafts has been difficult to highlight and is still a matter of debate.6 Although some studies have suggested that NK cells do not participate to rejection of solid organ transplants,7,8 it has been recently shown that, in certain models, NK cells could function as potent effector cells to promote allograft rejection9 or to trigger allograft vasculopathy.10 A dominant role of NK cells in fully allogeneic cardiac transplant rejection was reported in CD28-deficient recipients with impaired T-cell costimulatory functions. This effect of NK cells was attributed to their capacity to promote allogeneic dendritic cell (DC) maturation and subsequent alloreactive T-cell activation, overcoming the CD28 deficiency.9,11 This hypothesis, however, has been recently challenged by the demonstration, that in normal mice, host NK cells were implicated in the induction of islet allograft tolerance through costimulation blockade.12 Although the mechanisms were not defined, it was shown that NK cell–derived perforin (pfp) was required for tolerance induction.12 Likewise, a prominent role for NK cells has been recently reported in a skin-graft model of transplantation tolerance induced by costimulator blockade.13 Interestingly, it was shown that host NK cells were able to kill graft-derived antigen presenting cells (APCs), including DCs, thereby preventing excessive priming of alloreactive T cells in lymphoid and nonlymphoid tissues. These data indicate that, in the absence of NK cells, allogeneic DCs can persist in the host and enhance T-cell priming, thereby hindering the induction of tolerance to these highly immunogenic transplants.13 This observation is in agreement with our previous work showing that recognition of allogeneic DCs by host NK cells strongly affected the magnitude of allospecific CD4+ T-cell responses as well as their cytokine secretion profiles.14
Altogether, these observations suggested that host NK cells, through their ability to recognize and kill cells expressing allogeneic MHC class I molecules, might limit the persistence of donor-derived DCs, thereby inhibiting alloreactive T-cell priming through the direct pathway.13,14 As we recently showed that host allospecific CTLs can rapidly eliminate semi-allogeneic DCs in lymph nodes,15 we have used CD8α-deficient mice as recipients.14 In this model, we examined the site and the mechanism of allogeneic DC elimination by host NK cells, and its consequences on alloreactive CD4+ and CD8+ T-cell responses.
Methods
Mice
BALB/c (H-2d) and C57BL/6 (B6) (H-2b) mice were purchased from Center d'Elevage R. Janvier (Le Genest St Isle, France). β2-microglobulin−/− mice on the BALB/c background were generated as previously described.14 B6 CD8α−/−, B6 perforin-deficient (pfp−/−), and B6 IL-12Rβ2−/− mice were initially obtained from The Jackson Laboratory (Bar Harbor, ME). B6 CD8α−/−, (BALB/c x B6)F1 (CB6 F1), B6 pfp−/−, B6 CD8α−/−pfp−/−, B6 IL-12Rβ2−/−, and BALB/c β2-microglobulin−/− mice were bred and maintained in our specific pathogen-free animal facility. All experiments involving animals were performed in compliance with the relevant laws and institutional guidelines: Inserm approval no. 31-167, ethical review no. MP/03/05/03/04.
In vivo monoclonal antibody treatment
For in vivo NK-cell depletion, the anti-NK1.1 PK136 mouse IgG2a monoclonal antibody (mAb) (HB 191, ATCC, Manassas, VA) was purified from ascites fluid by caprylic acid precipitation. Mice were injected intraperitoneally with 200 μg of PK136 the day before and the day of immunization. For specific NK-cell subset depletion, 100 μg of the anti-Ly49D 4E5 rat IgG2a mAb or 100 μg of anti-Ly49A/D 12A8 rat IgG2a mAb was injected at days −1 and 0 of the experiment. For in vivo CD8+ T-cell depletion, mice were injected with anti-CD8α 53–6.72 rat IgG2a (TIB-105, ATCC) as previously shown.15 For L-selectin blockade experiments, mice received 100 μg of anti-CD62L monoclonal antibody Mel-14 3 hours before DC injection.
Skin grafting and histologic studies
Skin grafts were performed as previously described.14 For histologic analysis, skin grafts were fixed with formol for 24 hours at 37°C, and then tissue sections were stained with hematoxylin and eosin, after paraffin embedding. The percentage of eosinophils among infiltrating mononuclear cells was determined in at least 3 fields per graft as previously shown.15
Bone marrow-derived dendritic cells
Mouse bone marrow cells were cultured in complete culture medium at 2 × 106 in bacteriologic Petri dish (Greiner Bio-one, Frickenhausen, Germany) supplemented with supernatant of J558L cells transfected with a plasmid expressing the murine granulocyte macrophage-colony stimulating factor (GM-CSF). The method used here was adapted from a protocol previously described.16 DC preparations (> 80% of CD11cpos cells) were used at day 8 or 9 of culture to immunize mice subcutaneously into the hind footpads.
T-cell assays
CD4+ T cells from popliteal and inguinal lymph nodes of DC-immunized mice or inguinal and axillary lymph nodes draining the skin graft were purified by negative selection as previously described.15 CD4+ T cells (purity > 90%) were cultured with irradiated allogeneic splenocytes in HL-1 synthetic medium (Cambrex, Walkersville, MD). Supernatants were collected after 72 hours for cytokine analysis. Interferon-γ (IFN-γ) and interleukin-4 (IL-4) were quantified by 2-site sandwich enzyme-linked immunosorbent assay as previously described.17 For T-cell proliferation assays, cell cultures were pulsed the last 8 hours of culture with 1 μCi (37 KBq) [3H]TdR (40 Ci/nmol; the Radiochemical Center, GE Healthcare, Little Chalfont, United Kingdom). Incorporation of [3H]TdR was measured by an MicroBeta TriLux luminescence counter (PerkinElmer Life and Analytical Sciences, Waltham, MA).
Flow cytometry
For the analysis of intracellular cytokine synthesis, CD4+ T cells were cultured in complete medium with T cell–depleted allogeneic BALB/c splenocytes for 8 hours in the presence of anti-CD28 mAb (3 μg/mL, e-Bioscience, San Diego, CA) as previously described.15 Brefeldin A (Sigma-Aldrich, St Louis, MO) was added at 10 μg/mL for the last 4 hours of culture. The following mAbs purchased from BD Biosciences PharMingen (San Diego, CA) were used: anti-CD4, anti-CD69, and anti-IL-4 (11B11 clone). For NK-cell phenotyping, the following fluorochrome-conjugated mAbs (BD Biosciences PharMingen) were used: anti–TCR-β, anti-NK1.1, anti-DX5, anti-CD11b, anti-CD69, anti-CD127, and anti-Ly49D (4E5 clone). Intracellular staining for IFN-γ was performed on ex vivo isolated lymph node cells as described elsewhere.18
DC migration assay
For in vivo cytotoxic assay, B6 and BALB/c DCs were labeled with either 1 μM or 10 μM of carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA) as described15 and then injected subcutaneously into the hind footpads. At indicated times, draining popliteal lymph nodes were removed and digested with collagenase IV (Sigma-Aldrich) at 400 U/mL for 30 minutes at 37°C. The following fluorochrome-conjugated mAbs were used: anti-CD11c (BD Biosciences PharMingen) and anti-MHC class II (e-Bioscience). Cells were gated on CD11cpos, MHC IIhigh, and CFSEpos cells that represent approximately 2% to 4% of CD11cpos MHC IIhigh DCs in lymph nodes. Percentages of allogeneic target BALB/c cells were normalized to control syngeneic B6 cells. All cytometric data were collected on a FACSCalibur (BD Biosciences, San Jose, CA) and analyzed using the FlowJo software (Tree Star, Olten, Switzerland).
Confocal microscopy
DCs from indicated origins were labeled with 10 μM of CFSE or 10 μM of (5-(and -6-)-((4-chloromethyl)benzoyl)amino) tetramethylrhodamine (CMTMR; Invitrogen) as indicated by the manufacturer. Depending on the experiment, CMTMR-labeled BALB/c cells alone or a mixture of 106 CFSE-labeled BALB/c DCs and 106 CMTMR-labeled B6 DCs were injected subcutaneously into the hind footpads of recipient mice. At indicated times after injection, popliteal draining lymph nodes were harvested and prepared for confocal microscopic analysis as previously described.15 Cryostat sections (10 μm) were stained with unconjugated antiperipheral lymph node addressin (PNAd) mAb (Meca-79, BD Biosciences PharMingen) and anti-B220 mAb (RA36B2, BD Biosciences PharMingen). PNAd and B220 were revealed with AlexaFluor647 goat anti–rat Ig antibodies (Invitrogen). In some experiments, frozen sections of lymph nodes were fixed with acetone and stained with goat anti-NKp46 antibodies (R&D Systems, Minneapolis, MN), followed by donkey anti–goat antibodies conjugated to Alexa 488 or Alexa 633 (Invitrogen). Sections were analyzed with a LSM 510 confocal microscope and acquisition system (Carl Zeiss, Jena, Germany) equipped with a 10×/1.4 objective lens. Images were then processed with Adobe Photoshop CS2 software version 8.0.1 (Adobe Systems, Mountain View, CA).
Statistical analysis
Differences between variables were evaluated with by the Student t test using Prism GraphPad software (San Diego, CA).
Results
Effect of host NK-cell activation on allospecific CD4+ T-cell development and skin allograft rejection in vivo
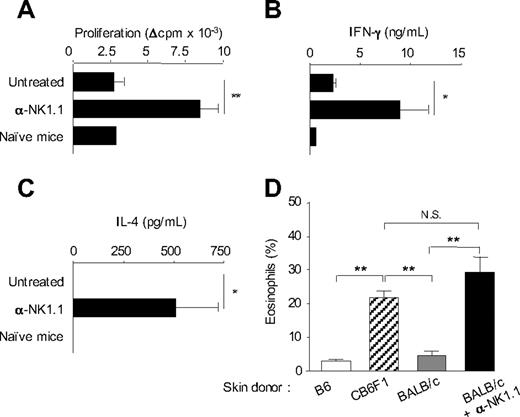
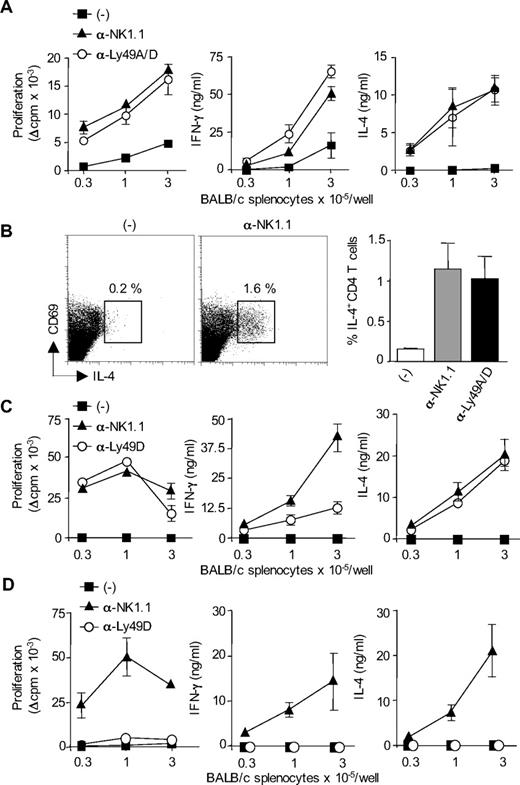
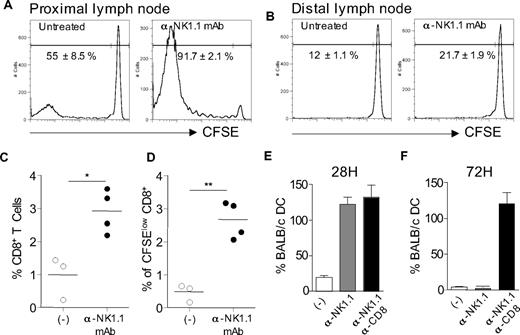
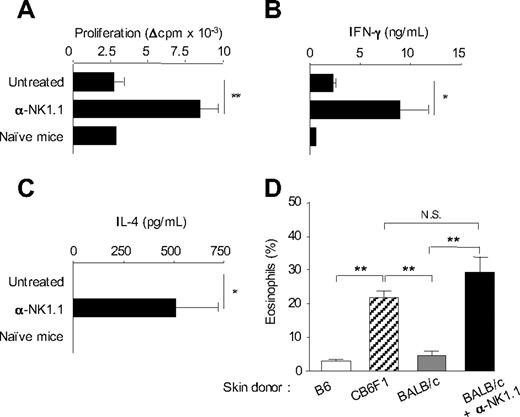
We previously showed that activation of host NK cells by allogeneic DCs immunization or skin graft transplantation, in recipient mice lacking CD8+ T cells, resulted in diminished allospecific Th cell responses associated with the development of effector Th cells producing IFN-γ but no type 2 cytokines. NK-cell elimination was sufficient to restore strong alloreactive CD4+ T-cell priming and to promote Th2 cell development.14 A typical experiment is presented in Figure 1, where CD8-deficient B6 mice, depleted or not of NK cells by anti-NK1.1 treatment, were grafted with fully allogeneic BALB/c skin. At day 8, CD4+ T cells were purified from draining lymph nodes and stimulated in vitro with allogeneic BALB/c APCs. In agreement with previous work,14 CD4+ T cells from NK-depleted mice proliferated vigorously and produced large amounts of IFN-γ and IL-4. By contrast, in presence of NK cells, priming with allogeneic skin graft induced a modest proliferative response of alloreactive CD4+ T cells that produced some IFN-γ but no IL-4 (Figure 1B,C). Histologic analysis of rejected allogeneic BALB/c skin grafts showed massive eosinophil infiltrates in NK cell–depleted mice, confirming the Th2 bias of the alloreactive response (Figure 1D). As a control to prevent NK-cell activation by donor allograft, we used (BALB/c x B6)F1 mice, which express recipient MHC I molecules as skin graft donors. As expected, in this combination, skin graft rejection was associated with strong tissue eosinophilia (Figure 1D) and alloreactive Th2 cell priming (data not shown). Taken together, our data show that in absence of NK-cell activation, CD4+ T cell–mediated graft rejection is characterized by enhanced expansion of alloreactive effector T lymphocytes, including Th2 cells probably responsible for the massive eosinophilic infiltration in rejected skin allografts.
In the absence of NK-cell activation, allogeneic skin grafting induces strong alloreactive CD4+ T-cell responses associated with Th2 cell development and massive eosinophil recruitment. (A-C) B6 CD8−/− mice, treated or not with anti-NK1.1 PK136 mAb, received a skin graft from allogeneic BALB/c mice. Eight days after grafting, draining lymph nodes were harvested. Lymph nodes from naive mice were taken as control. Purified CD4+ T cells were cultured (2 × 105 cells/well) in the presence of irradiated allogeneic BALB/c splenocytes (1 × 105 cells/well) for 72 hours. (A) CD4+ T-cell proliferation was evaluated by 3H-TdR incorporation. Background proliferation was less than 500 cpm. (B) IFN-γ and (C) IL-4 productions were measured by enzyme-linked immunosorbent assay in 72-hour culture supernatants. Data are mean plus or minus SEM of 5 mice per group. (D) B6 CD8−/− mice, treated or not with anti-NK1.1, received a skin graft from syngeneic B6, semi-allogeneic CB6F1, or fully allogeneic BALB/c mice. At the day of graft rejection (days 10-12), transplanted skins were harvested. Skin histologic analyses were performed, and the percentage of eosinophils among total mononuclear infiltrating cells was evaluated (3-5 mice per group). Data are from one representative experiment of at least 2 performed. *P < .05. **P < .01. N.S. indicates not significant.
In the absence of NK-cell activation, allogeneic skin grafting induces strong alloreactive CD4+ T-cell responses associated with Th2 cell development and massive eosinophil recruitment. (A-C) B6 CD8−/− mice, treated or not with anti-NK1.1 PK136 mAb, received a skin graft from allogeneic BALB/c mice. Eight days after grafting, draining lymph nodes were harvested. Lymph nodes from naive mice were taken as control. Purified CD4+ T cells were cultured (2 × 105 cells/well) in the presence of irradiated allogeneic BALB/c splenocytes (1 × 105 cells/well) for 72 hours. (A) CD4+ T-cell proliferation was evaluated by 3H-TdR incorporation. Background proliferation was less than 500 cpm. (B) IFN-γ and (C) IL-4 productions were measured by enzyme-linked immunosorbent assay in 72-hour culture supernatants. Data are mean plus or minus SEM of 5 mice per group. (D) B6 CD8−/− mice, treated or not with anti-NK1.1, received a skin graft from syngeneic B6, semi-allogeneic CB6F1, or fully allogeneic BALB/c mice. At the day of graft rejection (days 10-12), transplanted skins were harvested. Skin histologic analyses were performed, and the percentage of eosinophils among total mononuclear infiltrating cells was evaluated (3-5 mice per group). Data are from one representative experiment of at least 2 performed. *P < .05. **P < .01. N.S. indicates not significant.
Ly49D+ NK cells regulate alloreactive CD4+ T-cell priming and polarization in response to allogeneic H-2d DCs
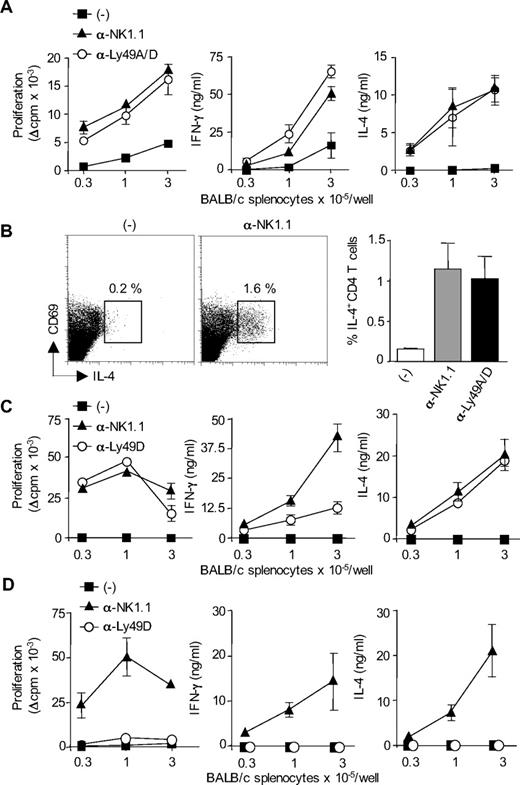
We next thought to determine the subsets of host NK cells that are required for the regulation of H-2d-specific CD4+ T-cell responses in CD8-deficient B6. Because alloreactive T-cell response in this model of skin graft rejection is probably induced by donor DCs that migrate to the draining lymph nodes,19 CD8-deficient B6 mice were immunized with allogeneic BM-DCs from BALB/c mice. In this experimental setting, NK-cell depletion induced alloreactive CD4+ T-cell priming in allogeneic DC-immunized but not naive mice (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). As it has been reported in H-2d bone marrow transplant rejection,20 we hypothesized that the regulatory role of NK cells was mediated by a subset of NK cells expressing Ly49D activating receptor specific for H-2Dd MHC class I molecules expressed on allogeneic DCs.21,22 As shown in Figure 2A, administration of anti-Ly49A/D (12A8) mAb enhanced H-2d-specific proliferative CD4+ T-cell response and induced the development of effector Th cells producing not only IFN-γ but also IL-4. In contrast, allogeneic DC immunization of control B6 CD8−/− mice induced a moderate priming of alloreactive CD4+ T-cell responses. This was confirmed by analyzing the frequency of H-2d–specific Th2 cells by intracellular cytokine staining. IL-4 producing CD69+ CD4+ T cells specific for H-2d MHC class II molecules were detected at similar frequency in anti-NK.1.1 and anti-Ly49D/A–treated mice and represented approximately 1% of total CD4+ T lymphocytes. The frequency of IL-4+ CD4+ T cells was below 0.02% when T cells were cultured with syngeneic APCs (not shown). In NK sufficient mice immunized with allogeneic DCs (Figure 2B), the frequency of IL-4–producing CD4+ T cells was either very low (< 0.2%) or undetectable (not shown). Because 12A8 mAb depletes in addition to Ly49D+ cells a population of NK cells expressing the Ly49A inhibitory receptor, we evaluated the effect of selective depletion of Ly49D using the 4E5 mAb. In the spleen and lymph nodes of 12A8- or 4E5-treated mice, we noticed a slight reduction of NK- cell numbers and a complete disappearance of NK cells expressing high levels of Ly49D (Figure S2). This selective elimination of Ly49D-bearing NK cells had almost similar effect as complete NK- cell depletion, by restoring strong alloreactive CD4+ T-cell priming and expansion of IFN-γ and IL-4-producing effector Th cells (Figure 2C). We also evaluated the effect of Ly49D+ NK-cell depletion on CD4+ T-cell responses induced in B6 CD8−/− mice immunized with MHC class I–deficient BALB/c DCs. As shown in Figure 2D, β2-microglobulin–deficient BALB/c DCs were unable to prime alloreactive CD4+ T-cell responses in both control and anti-Ly49D–treated mice. By contrast, when recipient mice were depleted of their whole NK cell compartment using anti-NK1.1 mAb, a potent alloreactive H-2d-specific CD4+ T-cell response developed, characterized by a strong production of type 1 and type 2 effector cytokines (Figure 2D). Thus, in 4E5-treated mice, the remaining Ly49D− NK cells were still efficient in inhibiting alloreactive CD4+ T-cell priming by allogeneic β2-microglobulin–deficient BALB/c DCs. Similar results were obtained using anti-Ly49D/A mAb 12A8 (not shown). Altogether, these data indicate that the failure of allogeneic H-2d DCs to efficiently prime alloreactive CD4 T cells is a consequence of the activation of H-2Dd-specific Ly49D+ NK cells. These results also rule out any role for NKT cells as Ly49D molecules have indeed been reported to be exclusively expressed on NK cells.23-25
Ly49D+ NK cells regulate alloreactive CD4 T-cell priming and polarization in response to allogeneic H-2d DCs. (A) B6 CD8−/− mice, treated or not with anti-NK1.1 PK136 mAb or anti-Ly49A/D 12A8 mAb, were injected subcutaneously with allogeneic BALB/c DCs. Six days after immunization, CD4+ T cells were purified from draining lymph nodes and restimulated (2 × 105 cells/well) with irradiated allogeneic BALB/c splenocytes for 72 hours to measure proliferation and cytokine production. Data are mean plus or minus SEM of 3 mice per group. Data are from one representative experiment of 3 performed. (B) To evaluate the frequency of IL-4-producing cells, purified CD4+ T cells were cultured for 8 hours with T cell–depleted BALB/c splenocytes in the presence of anti-CD28 mAb. Intracytoplasmic staining for IL-4 was then performed. Data are percentage of CD69pos CD4pos T cells producing IL-4 (mean ± SEM of 3 mice per group). (C,D) B6 CD8−/− mice, treated or not with anti-NK1.1 PK136 mAb or anti-Ly49D 4E5 mAb, were injected subcutaneously with DCs derived from either WT BALB/c mouse (C) or β2-microglobulin−/− BALB/c mouse (D). Six days after immunization, CD4+ T cells were purified from draining lymph nodes and restimulated (2 × 105 cells/well) with irradiated allogeneic BALB/c splenocytes for 72 hours to measure proliferation and cytokine production. Data are mean plus or minus SEM of 3 mice per group. Background proliferation was less than 1500 cpm. Data are representative of at least 2 experiments performed.
Ly49D+ NK cells regulate alloreactive CD4 T-cell priming and polarization in response to allogeneic H-2d DCs. (A) B6 CD8−/− mice, treated or not with anti-NK1.1 PK136 mAb or anti-Ly49A/D 12A8 mAb, were injected subcutaneously with allogeneic BALB/c DCs. Six days after immunization, CD4+ T cells were purified from draining lymph nodes and restimulated (2 × 105 cells/well) with irradiated allogeneic BALB/c splenocytes for 72 hours to measure proliferation and cytokine production. Data are mean plus or minus SEM of 3 mice per group. Data are from one representative experiment of 3 performed. (B) To evaluate the frequency of IL-4-producing cells, purified CD4+ T cells were cultured for 8 hours with T cell–depleted BALB/c splenocytes in the presence of anti-CD28 mAb. Intracytoplasmic staining for IL-4 was then performed. Data are percentage of CD69pos CD4pos T cells producing IL-4 (mean ± SEM of 3 mice per group). (C,D) B6 CD8−/− mice, treated or not with anti-NK1.1 PK136 mAb or anti-Ly49D 4E5 mAb, were injected subcutaneously with DCs derived from either WT BALB/c mouse (C) or β2-microglobulin−/− BALB/c mouse (D). Six days after immunization, CD4+ T cells were purified from draining lymph nodes and restimulated (2 × 105 cells/well) with irradiated allogeneic BALB/c splenocytes for 72 hours to measure proliferation and cytokine production. Data are mean plus or minus SEM of 3 mice per group. Background proliferation was less than 1500 cpm. Data are representative of at least 2 experiments performed.
Increased recruitment and activation of host NK cells in allogeneic DC-draining lymph nodes
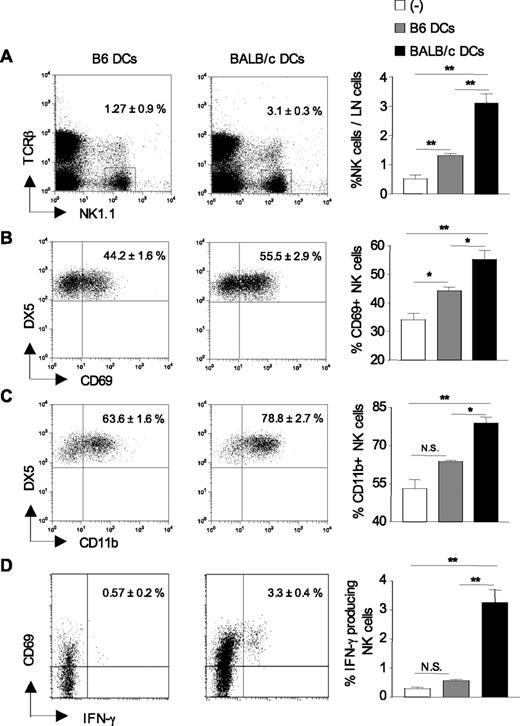
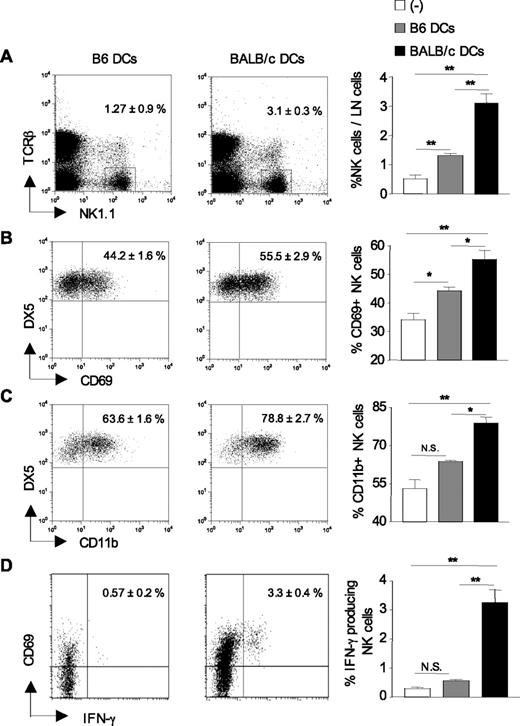
Having shown that Ly49D+ NK cells regulate CD4+ T-cell priming in response to allogeneic BALB/c DCs, we next examined the recruitment and activation status of host NK cells in DC-draining lymph nodes 24 hours after immunization. As shown in Figure 3A, the frequency of NK1.1+TCR-β− NK cells was increased up to 6-fold in allogeneic DC-draining lymph nodes compared with control lymph nodes from nonimmunized mice. Immunization with syngeneic B6 DCs also induced the recruitment of NK cells to draining lymph nodes, although to a lesser extent than fully allogeneic BALB/c DCs. Lymph node cellularity was equivalently enhanced in mice primed with syngeneic or allogeneic BALB/c DCs with similar proportion of T and B cells (not shown). NK-cell recruitment was associated with an increased frequency of NK cells expressing the activation markers CD69 (Figure 3B) or CD11b (Figure 3C) in allogeneic DC-draining lymph nodes compared with lymph nodes from control or syngeneic DC-immunized mice. Likewise, the frequency of IFN-γ-secreting CD69+NK1.1+TCR-β− NK cells increased from 0.2% to 3% or 4% at 24 hours after immunization (Figure 3D). These data collectively demonstrate that on immunization with allogeneic DCs, NK cells harboring an activated phenotype are rapidly recruited to draining lymph nodes.
Increased recruitment and activation of host NK cells in lymph nodes after allogeneic DC immunization. B6 CD8−/− mice were left untreated (−) or injected with either syngeneic B6 DCs or allogeneic BALB/c DCs. Twenty-four hours after immunization, draining lymph nodes were harvested, and the percentage of TCR-βneg NK1.1pos cells was determined (A) as well as the percentage of NK cells (TCR-βneg NK1.1posDX5pos) expressing CD69 (B) and CD11b (C). (D) The frequency of CD69pos NK cells (TCR-βneg NK1.1pos) producing IFN-γ was evaluated by ex vivo intracytoplasmic staining without prior stimulation. Data are mean plus or minus SEM of 4 or 5 mice per group. *P < .05. **P < .01. N.S. indicates not significant. Data are from one representative experiment of 3 performed.
Increased recruitment and activation of host NK cells in lymph nodes after allogeneic DC immunization. B6 CD8−/− mice were left untreated (−) or injected with either syngeneic B6 DCs or allogeneic BALB/c DCs. Twenty-four hours after immunization, draining lymph nodes were harvested, and the percentage of TCR-βneg NK1.1pos cells was determined (A) as well as the percentage of NK cells (TCR-βneg NK1.1posDX5pos) expressing CD69 (B) and CD11b (C). (D) The frequency of CD69pos NK cells (TCR-βneg NK1.1pos) producing IFN-γ was evaluated by ex vivo intracytoplasmic staining without prior stimulation. Data are mean plus or minus SEM of 4 or 5 mice per group. *P < .05. **P < .01. N.S. indicates not significant. Data are from one representative experiment of 3 performed.
Host Ly49D+ NK cells are responsible for allogeneic DC elimination in vivo
Thereafter, we investigated whether this rapid afflux of alloreactive NK cells could have an impact on allogeneic DC elimination in draining lymph nodes. We therefore monitored the accumulation and/or persistence of BALB/c DCs in the recipient lymph nodes. To this end, B6 CD8−/− mice were immunized subcutaneously with equal numbers of syngeneic B6 and allogeneic BALB/c DCs, labeled with low or high concentrations of CFSE dye, respectively. Histograms in Figure 4A are representative of CFSE-labeled DC profiles obtained at 48 hours from untreated and anti-NK1.1 treated B6 CD8−/− mice. As shown in Figure 4B, depletion of host NK cells was associated with an increase in allogeneic DC numbers in draining lymph nodes from 48 to 72 hours after immunization. Next, to determine whether Ly49D+ NK cells were implicated in the inhibition of allogeneic DC persistence, CD8-deficient B6 mice were treated, or not, with anti-NK.1.1 or anti-Ly49D mAb and then immunized with the same DC mixture as before. We found that allogeneic DC persistence was similarly increased in mice depleted of NK1.1+ or Ly49D+ NK cells, suggesting that allogeneic DC elimination was mediated by a subset of NK cells bearing the H-2Dd–specific Ly49D activating receptor (Figures 4C, S3).
The persistence of donor-derived DCs in lymph nodes is impaired in the presence of host Ly49D+ NK cells. (A,B) B6 CD8−/− mice, treated or not with anti-NK1.1 PK136 mAb, were injected with CFSElow-labeled syngeneic B6 DCs and CFSEhigh-labeled allogeneic BALB/c DCs. Draining lymph nodes were harvested at indicated times after immunization and analyzed for the presence of CFSE-labeled cells. Cells were gated on CD11cpos, MHC IIhigh, and CFSEpos cells. (A) Representative CFSE profiles of injected DCs obtained at 48 hours from untreated or PK136-treated mice. (A,B) Numbers in dot plots and histograms indicate the percentages of allogeneic BALB/c CFSEhigh cells normalized to control syngeneic B6 CFSElow cells [(%BALB/c DCs:%B6 DCs) × 100]. (C) B6 CD8−/− mice were treated, or not, with anti-NK1.1 PK136 mAb or anti-Ly49D 4E5 mAb, and injected as in panel A with a mixture of CFSElow-B6 DCs and CFSEhigh-BALB/c DCs. Forty-eight hours after immunization, the presence of CFSE-positive cells was analyzed in the draining lymph nodes, and the percentage of CFSEhigh BALB/c cells among control CFSElow B6 cells was determined. Data are mean plus or minus SEM of 3 or 4 mice per group. (D) B6 CD8−/− mice treated, or not, with PK136 were injected with 1 or 2 × 106 CMTMR-labeled BALB/c DCs. Forty-eight hours after immunization, draining lymph nodes were harvested, prepared and stained with anti-B220 and anti-PNAd mAbs (blue). Lymph nodes sections were then analyzed by confocal microscopy, and the numbers of red DCs per lymph node were evaluated. Data are mean plus or minus SEM of 4 lymph nodes per group (2 mice/group). (E,F) B6 CD8−/− mice were treated or not with PK136 as indicated before immunization with DCs. (E) Mice were immunized with CMTMR-labeled BALB/c DCs (red). Frozen sections of lymph nodes at 48 hours were stained with goat anti-NKp46 antibodies and then donkey anti–goat Alexa488 antibodies. Numbers of NK cells and BALB/c DCs per section were counted. Data are mean plus or minus SEM of 4 to 6 lymph nodes per group. In panel F, mice were injected with CFSE-labeled BALB/c DCs (green) and CMTMR-labeled B6 DCs (red). Draining lymph nodes were removed at indicated times after immunization to evaluate the kinetics of allogeneic DC appearance. Lymph node sections were then stained with anti-B220 and anti-PNAd mAbs (blue). Representative tissue sections from untreated or PK136-treated mouse lymph nodes harvested at 48 hours after injection are depicted. Histograms indicate the percentage of CFSE BALB/c cells among control CMTMR B6 cells. Data are mean plus or minus SEM of individual lymph nodes (2 or 3 mice per group). *P < .05. **P < .01. N.S. indicates not significant. Data are representative of at least 2 or 3 experiments performed.
The persistence of donor-derived DCs in lymph nodes is impaired in the presence of host Ly49D+ NK cells. (A,B) B6 CD8−/− mice, treated or not with anti-NK1.1 PK136 mAb, were injected with CFSElow-labeled syngeneic B6 DCs and CFSEhigh-labeled allogeneic BALB/c DCs. Draining lymph nodes were harvested at indicated times after immunization and analyzed for the presence of CFSE-labeled cells. Cells were gated on CD11cpos, MHC IIhigh, and CFSEpos cells. (A) Representative CFSE profiles of injected DCs obtained at 48 hours from untreated or PK136-treated mice. (A,B) Numbers in dot plots and histograms indicate the percentages of allogeneic BALB/c CFSEhigh cells normalized to control syngeneic B6 CFSElow cells [(%BALB/c DCs:%B6 DCs) × 100]. (C) B6 CD8−/− mice were treated, or not, with anti-NK1.1 PK136 mAb or anti-Ly49D 4E5 mAb, and injected as in panel A with a mixture of CFSElow-B6 DCs and CFSEhigh-BALB/c DCs. Forty-eight hours after immunization, the presence of CFSE-positive cells was analyzed in the draining lymph nodes, and the percentage of CFSEhigh BALB/c cells among control CFSElow B6 cells was determined. Data are mean plus or minus SEM of 3 or 4 mice per group. (D) B6 CD8−/− mice treated, or not, with PK136 were injected with 1 or 2 × 106 CMTMR-labeled BALB/c DCs. Forty-eight hours after immunization, draining lymph nodes were harvested, prepared and stained with anti-B220 and anti-PNAd mAbs (blue). Lymph nodes sections were then analyzed by confocal microscopy, and the numbers of red DCs per lymph node were evaluated. Data are mean plus or minus SEM of 4 lymph nodes per group (2 mice/group). (E,F) B6 CD8−/− mice were treated or not with PK136 as indicated before immunization with DCs. (E) Mice were immunized with CMTMR-labeled BALB/c DCs (red). Frozen sections of lymph nodes at 48 hours were stained with goat anti-NKp46 antibodies and then donkey anti–goat Alexa488 antibodies. Numbers of NK cells and BALB/c DCs per section were counted. Data are mean plus or minus SEM of 4 to 6 lymph nodes per group. In panel F, mice were injected with CFSE-labeled BALB/c DCs (green) and CMTMR-labeled B6 DCs (red). Draining lymph nodes were removed at indicated times after immunization to evaluate the kinetics of allogeneic DC appearance. Lymph node sections were then stained with anti-B220 and anti-PNAd mAbs (blue). Representative tissue sections from untreated or PK136-treated mouse lymph nodes harvested at 48 hours after injection are depicted. Histograms indicate the percentage of CFSE BALB/c cells among control CMTMR B6 cells. Data are mean plus or minus SEM of individual lymph nodes (2 or 3 mice per group). *P < .05. **P < .01. N.S. indicates not significant. Data are representative of at least 2 or 3 experiments performed.
Then, to directly visualize allogeneic DC persistence in draining lymph nodes in absence of host NK cells, we performed confocal microscopy analysis. B6 CD8−/− mice were injected with BALB/c DCs labeled with CMTMR (red). As shown in Figure 4D,E, allogeneic DCs were present at high numbers only in NK-depleted mice. NK cells were then visualized by NKp46 staining.26 Consistent with previous work,18,26 NK cells were found in the T-cell zone of lymph nodes of control but not anti-NK1.1–treated mice (Figure 4E) and therefore colocalized in the same lymph node areas as DCs (Figures S5, S7). We next determined the kinetics of DC elimination in the draining lymph nodes by injecting B6 CD8−/− mice with a mixture of CFSE-labeled BALB/c DCs (green) and CMTMR-labeled B6 DCs (red). Data in Figure 4F show that allogeneic DC numbers were not affected by the presence or absence of NK cells at an early time point (18 hours). By contrast, alterations in BALB/c DC numbers were evident at 24 to 48 hours in control mice compared with NK-depleted hosts. Altogether, these data show that allogeneic DCs fail to accumulate and to persist in the presence of NK cells, and suggest that NK cells expressing the Ly49D activating receptor participate in a large extent to the rapid elimination of allogeneic DCs within the draining lymph nodes.
Recently recruited NK cells are responsible of allogeneic DC killing
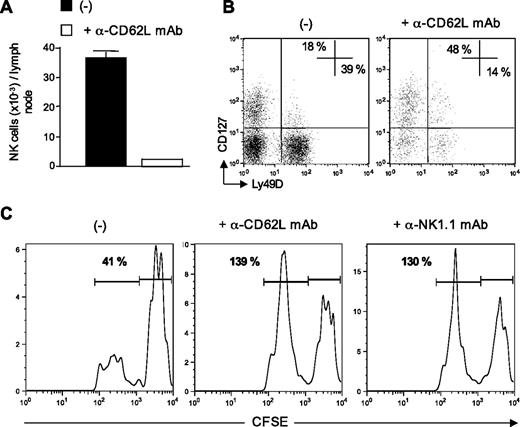
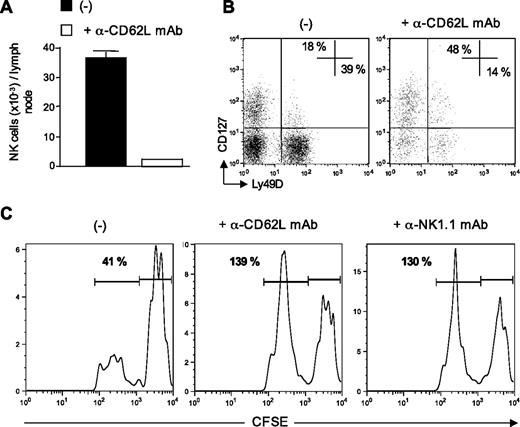
Data from Figures 3 and 4 indicated that the rapid afflux of NK cells in the draining lymph nodes was associated with the elimination of donor-derived allogeneic DCs. We therefore investigated whether newly recruited NK cells rather than resident NKs were directly implicated in allogeneic DC killing. To assess this point, we performed L-selectin–blocking experiments as some reports have shown that entry of blood NK cells into lymph nodes was mediated through high endothelial venules in a CD62L-dependent manner.18,27,28 B6 CD8−/− mice were injected with anti-CD62L blocking mAb and immunized 3 hours later with allogeneic DCs. At 48 hours after DC injection, draining lymph nodes were harvested and analyzed for the presence of NK cells and allogeneic DCs. As expected, the number of NK cells recruited into DC-draining lymph nodes was reduced by 90% by injecting anti-CD62L mAb (Figure 5A). We looked at the repartition of 2 NK cell subsets, the newly described thymic NK cells expressing CD127 (IL-7 receptor α)29 and the conventional CD127− NK subset. We found that CD62L blockade preferentially affected the conventional subset with a marked decrease in the percentage of CD127− NK cells expressing Ly49D (Figure 5B). Blockade of blood-borne NK cell migration into the lymph nodes prevented allogeneic DC killing as efficiently as NK cell depletion (Figure 5C). These results strongly suggested that CD127− Ly49D+ NK cells recruited from the blood into the draining lymph nodes were responsible for allogeneic DC elimination, rather than lymph node-resident NK cells.
Recently recruited NK cells are responsible for allogeneic DC killing. (A-C) B6 CD8−/− mice, treated, or not, with anti-CD62L Mel-14 mAb, were immunized with CFSEhigh syngeneic B6 DCs and CFSElow allogeneic BALB/c DCs. Forty-eight hours after injection, draining lymph nodes were removed and analyzed for the presence of NK cells and CFSE DCs (CD11cpos MHC IIhigh CFSEpos cells). (A) Absolute numbers of NK cells (TCR-βneg NK1.1pos) recovered from lymph node are indicated. (B) Expression of CD127 and Ly49D on NK cells (TCR-βneg NK1.1pos). (C) Representative CFSE profiles of injected DCs from control, Mel-14–, or PK136-treated mouse lymph nodes are shown. Numbers indicate the percentage of CFSElow BALB/c DCs among control CFSEhigh B6 DCs. A representative experiment of 3 is shown.
Recently recruited NK cells are responsible for allogeneic DC killing. (A-C) B6 CD8−/− mice, treated, or not, with anti-CD62L Mel-14 mAb, were immunized with CFSEhigh syngeneic B6 DCs and CFSElow allogeneic BALB/c DCs. Forty-eight hours after injection, draining lymph nodes were removed and analyzed for the presence of NK cells and CFSE DCs (CD11cpos MHC IIhigh CFSEpos cells). (A) Absolute numbers of NK cells (TCR-βneg NK1.1pos) recovered from lymph node are indicated. (B) Expression of CD127 and Ly49D on NK cells (TCR-βneg NK1.1pos). (C) Representative CFSE profiles of injected DCs from control, Mel-14–, or PK136-treated mouse lymph nodes are shown. Numbers indicate the percentage of CFSElow BALB/c DCs among control CFSEhigh B6 DCs. A representative experiment of 3 is shown.
Perforin-mediated killing is critical for the elimination of allogeneic DCs by host NK cells in vivo
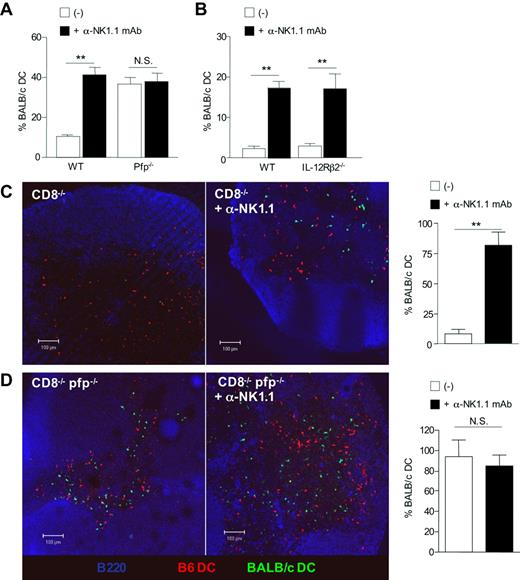
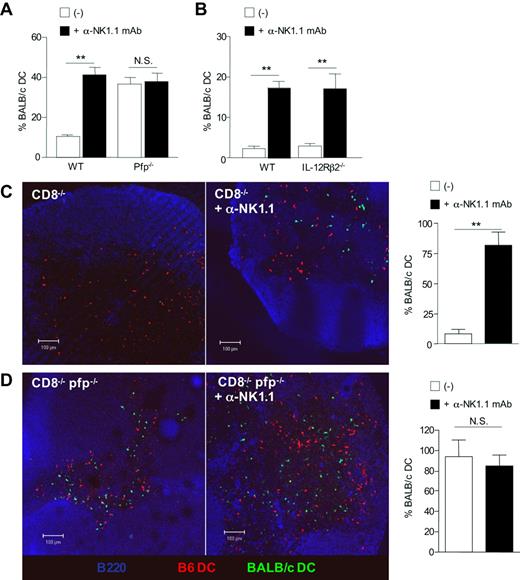
To gain insight into the mechanisms underlying allogeneic DC elimination by NK cells, we analyzed DC persistence in perforin-deficient mice, as perforin is a critical molecule in the cytolytic function of NK cells.30 Groups of B6 WT and B6 pfp−/− mice were all depleted of CD8+ T lymphocytes by antibody treatment, as we and others recently showed that CTL can rapidly kill semi-allogeneic DCs within the draining lymph nodes.15,31 Groups of mice received in addition anti-NK1.1 mAb before injection with equal numbers of allogeneic BALB/c and syngeneic B6 DCs differentially labeled with CFSE (Figures 6A, S4A). We found that allogeneic DCs failed to accumulate unless NK cells were previously depleted. In contrast, similar numbers of allogeneic DCs were found in both control and NK-depleted pfp-deficient B6 mice, suggesting that perforin-dependent cytotoxicity is the main pathway of NK cell–mediated allogeneic DC killing in vivo. Unlike for allogeneic DCs, migration and survival of syngeneic B6 DCs were not affected by perforin deficiency (not shown). We also investigated the role of IL-12R signaling in NK cells, as IL-12 has been reported to be required for NK-cell cytotoxicity in some in vivo models.32 As shown in Figures 6B and S4B, NK cells in IL-12Rβ2–deficient mice were as effective as in WT mice in mediating cytotoxic elimination of allogeneic DCs in vivo. The key role played by perforin in the elimination of allogenic DC was then confirmed by confocal microscopic analysis. B6 CD8−/− or B6 CD8−/−pfp−/− double-knockout mice were coinjected with BALB/c (green) and B6 (red) DCs (Figure 6C,D). Whereas allogeneic BALB/c DCs were almost absent in B6 CD8−/− mice (Figure 6C), they were readily detectable within the draining lymph nodes of B6 CD8−/−pfp−/− mice (Figure 6D), despite similar numbers of NK cells (Figure S5). Depletion of host NK cells by anti-NK.1.1 treatment fully restored allogeneic DC numbers in B6 CD8−/− mice (Figure 6C), whereas it had no significant effect on allogeneic DC persistence in perforin-deficient B6 CD8−/− mice (Figure 6D). Likewise, strong alloreactive CD4+ T-cell priming occurred in pfp-deficient B6 CD8−/− mice whether NK cells were present or not (Figure S6). To demonstrate that perforin in NK cells was indeed crucial for allogeneic DC elimination, we examined whether adoptively tranferred wild-type NK cells could influence BALB/c DCs numbers in perforin-deficient CD8−/− mice. Allogeneic BALB/c DC numbers were substantially reduced in lymph node sections from mice reconstituted with highly purified wild-type NK cells (Figure S7). Altogether, these data demonstrate that perforin-dependent cytotoxicity is the main pathway implicated in allogeneic DC elimination by host NK cells within lymph nodes.
Perforin-mediated killing is critical for the elimination of allogeneic DCs by host NK cells in vivo. (A) B6 WT, B6 pfp−/− or (B) IL-12Rβ2−/− mice, all CD8-depleted, were injected, or not, with anti-NK1.1 PK136 mAb and then immunized with equal numbers of CFSElow-B6 DCs and CFSEhigh-BALB/c DCs. (A) Data are mean plus or minus SEM of 3 mice per group and are from one representative experiment of 4 performed. (B) Data are mean plus or minus SEM of 4 mice per group. (C) B6 CD8−/− and (D) CD8−/−pfp−/− mice were injected with equal numbers of CFSE-labeled BALB/c DCs (green) and CMTMR-labeled B6 DCs (red). Forty-eight hours after immunization, draining lymph nodes were harvested and prepared for confocal microscopy analysis. Lymph node sections were stained anti-B220 and anti-PNAd mAbs (blue). Data are the percentage of CFSE BALB/c DCs among control CMTMR B6 DCs. Data were pooled from 2 independent experiments. Data are mean plus or minus SEM of individual lymph nodes (4 or 5 mice per group). **P < .01. N.S. indicates not significant.
Perforin-mediated killing is critical for the elimination of allogeneic DCs by host NK cells in vivo. (A) B6 WT, B6 pfp−/− or (B) IL-12Rβ2−/− mice, all CD8-depleted, were injected, or not, with anti-NK1.1 PK136 mAb and then immunized with equal numbers of CFSElow-B6 DCs and CFSEhigh-BALB/c DCs. (A) Data are mean plus or minus SEM of 3 mice per group and are from one representative experiment of 4 performed. (B) Data are mean plus or minus SEM of 4 mice per group. (C) B6 CD8−/− and (D) CD8−/−pfp−/− mice were injected with equal numbers of CFSE-labeled BALB/c DCs (green) and CMTMR-labeled B6 DCs (red). Forty-eight hours after immunization, draining lymph nodes were harvested and prepared for confocal microscopy analysis. Lymph node sections were stained anti-B220 and anti-PNAd mAbs (blue). Data are the percentage of CFSE BALB/c DCs among control CMTMR B6 DCs. Data were pooled from 2 independent experiments. Data are mean plus or minus SEM of individual lymph nodes (4 or 5 mice per group). **P < .01. N.S. indicates not significant.
Early killing of allogeneic H-2d DCs by host NK cells inhibits alloreactive CD8+ T-cell priming in vivo
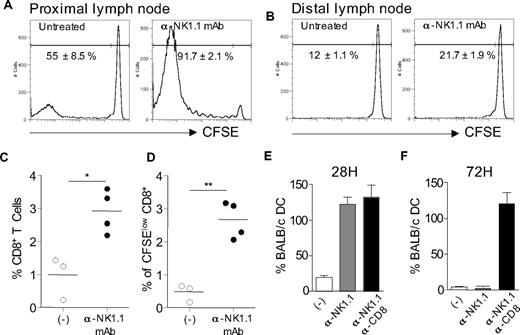
Because CD8+ T cells can clearly contribute to allogeneic DC killing in the lymph nodes,15 we used so far CD8-deficient mice to focus more accurately on the mechanisms of DC killing by NK cells. To assess the significance of our findings in a situation where other MHC class I–directed pathways are operating, we first evaluated the effect of NK cells on alloreactive CD8+ T cell-priming. For this, CD8-deficient mice were reconstituted with CFSE-labeled CD8+ T lymphocytes from normal mice before allogeneic DC immunization. Recipient mice were depleted or not of NK cells by anti-NK1.1 mAb treatment. CFSE dilution in CD8+ T cells in draining lymph nodes was evaluated 4 days later. In control mice, CD8+ T lymphocytes that had divided and thus demonstrated lower CFSE intensity were detected in the lymph nodes draining the allogeneic DC injection site (Figure 7A) compared with distal lymph nodes (Figure 7B) or lymph nodes from naive control (data not shown). Interestingly, NK-cell depletion further increased the frequency of proliferating CD8+ T cells (Figure 7A-D). We next evaluated the NK-cell requirement for allogeneic DC elimination in wild-type mice. Normal mice were either left untreated, depleted of NK cells, or both NK cells and CD8+ T lymphocytes before administration of differentially labeled DCs as in Figure 6. Draining lymph nodes were collected after 28 or 72 hours to determine the frequency of allogeneic BALB/c DCs by confocal microscopy. Data in Figure 7E,F show that, in normal mice, NK cells have already eliminated allogeneic DCs by 28 hours, before CD8+ T cell–mediated killing that occurred later by 48 to 72 hours, in agreement with our previous work.15 In conclusion, these data demonstrate that host NK cells can kill allogeneic H-2d DCs in the presence of CD8+ T cells, thereby limiting alloreactive CD8+ T-cell priming in vivo.
Host NK cells inhibit alloreactive CD8+ T-cell priming in vivo through their capacity to rapidly eliminate allogeneic H-2d DCs in draining lymph nodes. (A,B) B6 CD8−/− mice, treated, or not, with anti-NK1.1 PK136 mAb, were injected intravenously with 107 CFSE-labeled CD8+ T lymphocytes. Mice were then immunized with allogeneic BALB/c DCs (106/mouse) 1 day after passive CD8+ T-cell transfer. Four days after immunization, CFSE dilution in TCR-β+CD8+ T cells was analyzed in popliteal (proximal) or inguineal (distal) lymph node cells by flow cytometry (A). (C,D) Percentage of CD8+ T cells (C) and CFSE low CD8+ T cells (D) among total popliteal lymph node cells from individual mice (3 or 4 per group). Data are representative of 3 experiments performed. (E,F) Normal C57BL/6 mice were injected with anti-NK1.1 PK136 mAb alone or together with anti-CD8–depleting mAb as indicated. Mice were immunized with a mixture of CFSE-labeled BALB/c DCs (green) and CMTMR-labeled B6 DCs (red) as in Figure 6. Lymph nodes were collected at 28 hours (E) or 72 hours (F) and processed for confocal microscopic analysis as in Figure 6. Histograms indicate the percentage of CFSE BALB/c DCs among control CMTMR B6 DCs. Data are mean plus or minus SEM of individual lymph nodes (2 or 3 mice per group) and are representative of 2 experiments performed. *P < .05. **P < .01.
Host NK cells inhibit alloreactive CD8+ T-cell priming in vivo through their capacity to rapidly eliminate allogeneic H-2d DCs in draining lymph nodes. (A,B) B6 CD8−/− mice, treated, or not, with anti-NK1.1 PK136 mAb, were injected intravenously with 107 CFSE-labeled CD8+ T lymphocytes. Mice were then immunized with allogeneic BALB/c DCs (106/mouse) 1 day after passive CD8+ T-cell transfer. Four days after immunization, CFSE dilution in TCR-β+CD8+ T cells was analyzed in popliteal (proximal) or inguineal (distal) lymph node cells by flow cytometry (A). (C,D) Percentage of CD8+ T cells (C) and CFSE low CD8+ T cells (D) among total popliteal lymph node cells from individual mice (3 or 4 per group). Data are representative of 3 experiments performed. (E,F) Normal C57BL/6 mice were injected with anti-NK1.1 PK136 mAb alone or together with anti-CD8–depleting mAb as indicated. Mice were immunized with a mixture of CFSE-labeled BALB/c DCs (green) and CMTMR-labeled B6 DCs (red) as in Figure 6. Lymph nodes were collected at 28 hours (E) or 72 hours (F) and processed for confocal microscopic analysis as in Figure 6. Histograms indicate the percentage of CFSE BALB/c DCs among control CMTMR B6 DCs. Data are mean plus or minus SEM of individual lymph nodes (2 or 3 mice per group) and are representative of 2 experiments performed. *P < .05. **P < .01.
Discussion
Here we report that, in allotransplantation settings, host NK cells regulate alloreactive T-cell responses through direct killing of allogeneic DCs in secondary lymphoid organs. We showed that newly recruited Ly49D+ NK cells in B6 recipients specific for H-2 Dd MHC class I molecules were able to kill allogeneic BALB/c (H-2d) DCs through a perforin-dependent mechanism. Our data support a model in which donor-derived DCs migrate into the draining lymph nodes where they induce L-selectin–dependent recruitment of blood-borne NK cells. Thus, recruitment of alloreactive NK cells in graft draining lymph nodes could limit alloreactive T-cell priming through lysis of allogeneic DCs. Indeed, in a model of CD4+ T cell–mediated skin graft rejection, we showed that the absence of NK cell activation resulted in an enhanced expansion of alloreactive CD4+ T cells, including Th2 cells, which probably accounted for the massive infiltrates of eosinophils found in rejected allografts. In normal mice, we finally showed that NK cells quickly eliminated allogeneic DCs within the lymph nodes draining the DC injection site, thereby inhibiting alloreactive CD8+ T-cell responses. These data, in agreement with previous work,13,14 support the concept that host NK cells may suppress rather than enhance alloreactive CD4+ and CD8+ T-cell responses through their capacity to rapidly kill donor derived APCs in secondary lymphoid tissues.
Indeed, besides their important function in the elimination of transformed or infected cells in the periphery, recent evidence from both human and mouse studies has documented that NK cells could also regulate adaptive immune responses by modulating DC functions or by producing polarizing cytokines, such as IFN-γ.27,33-35 In vitro experiments have shown that human NK cells can efficiently kill autologous immature but not mature DCs, suggesting that NK cells might play an important regulatory role by selecting immunogenic mature DCs during the initiation of immune responses.33,34 The situation is different, however, in allotransplantation settings where mature allogeneic DCs, lacking self-MHC class I, should be readily eliminated by host alloreactive NK cells, leading to opposite effects on the T-cell responses. Indeed, donor NK alloreactivity has been exploited in bone marrow transplantation to improve clinical outcome. Alloreactive NK cells were not only able to induce more effective elimination of leukemia cells but also to prevent graft-versus-host disease through their capacity to kill recipient DCs, thereby avoiding alloreactive donor T-cell priming.36 Interestingly, in skin transplantation models, it has been recently shown that recipient NK cells were able to prevent the persistence of graft-derived allogeneic DCs. In this study, the presence of recipient NK cells was required to promote efficient allograft survival induced by costimulation blockade.13 However, the mechanisms by which NK cells were able to eliminate allogeneic DC in situ were not examined. Our present work now shows that encounter of mature allogeneic DCs with NK cells occurs in secondary lymphoid organs leading to NK cell–mediated killing of donor-derived DCs, thereby inhibiting alloreactive T-cell priming. In addition, we clearly established that the perforin pathway is mainly involved in allogeneic DC elimination. This is in contrast with the NK-mediated killing of immature autologous DC in vivo, which has been mainly attributed to TRAIL-mediated cytotoxicity rather than perforin.37
Phenotypically and functionally distinct subsets of NK cells have been described in human38 and in mouse.29 It is improbable that the immunoregulatory function of NK cells we observed in this work could be attributed to the lymph node resident CD127+ NK subset.29 In mouse lymph node resident, NK cells represent only 0.5% of mononuclear cells, of which only 20% are CD127+.29 However, although mouse NK cells appear to be excluded from lymph nodes under steady-state conditions, they can be recruited at high numbers into lymph node draining sites of immunization or infection.18,27,39,40 In a mouse model, it has been recently shown that mature DCs could promote NK-cell recruitment to lymph nodes, which provided an early source of IFN-γ required for optimal Th1 cell development.27 In our model, we showed that NK cells expressing Ly49D compose the main subset implicated not only in the regulation of T cell expansion and polarization but also in perforin-mediated allogeneic DC killing. Thus, the requirement for Ly49D expression together with pfp-dependent cytotoxicity indicated a role for CD127− rather than CD127+ NK subset. CD127− Ly49D+ NK cells are probably recruited from the peripheral blood into the DC-draining lymph nodes. In agreement with recent work,18,27 we showed that NK-cell recruitment in lymph nodes occurred through a L-selectin–dependent mechanism. Indeed, preventing lymphocyte recruitment by CD62L blockade resulted in an increased persistence of incoming allogeneic DCs, demonstrating that recently recruited NK cells were implicated in the perforin-dependent killing of these cells.
In conclusion, our study has characterized the mechanisms governing the killing of allogeneic DCs by host NK cells, which results in impaired development of alloreactive T-cell responses. Allogeneic (H-2d) DC elimination is perforin-dependent, occurs in draining lymph nodes, and requires the recruitment of CD127− Ly49D+ blood-borne NK cells. Our study, together with recent work,13 suggests that alloreactive NK cells through their capacity to rapidly kill donor-derived allogeneic DCs could synergize with immunosuppressive agents to limit T-cell alloreactivity and to improve induction of transplantation tolerance.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the personnel of the animal facility at the Institut Fédératif de Recherche 30 (IFR30) for skillful assistance; C. Coureau (INSERM U563), F. Capilla (Histopathology facility, IFR30), and Sophie Allart (Cellular Imaging facility, IFR30) for their technical assistance; Dr S. Guerder (INSERM U563) for stimulating discussions; and Dr H.R. MacDonald (Ludwig Institute for cancer research, Epalinges, Switzerland) and Dr T. Walzer (Center d'Immunologie de Marseille-Luminy, France) for kindly providing Mel-14 monoclonal antibody and anti-NKp46 antibodies, respectively.
This work was supported by a grant from Etablissement Français des Greffes and by institutional grants from INSERM. S.L., C.S., and J.D.C. were supported by fellowships from the Ministère de l'Education Nationale de la Recherche et des Technologies, and also from l'Association pour la Recherche sur le Cancer (S.L.) and la Fondation pour la Recherche Médicale (J.D.C.).
Authorship
Contribution: S.L. designed and performed research and analyzed data; C.S. performed research and analyzed data; J.D.C. designed research and analyzed data; J.O. provided reagents and reviewed the data and the manuscript; J.-C.G. designed research, analyzed data, and wrote the paper with input from the coauthors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Charles Guéry, INSERM U563, CHU Purpan, Place du Dr Baylac, BP 3028, 31 024 Toulouse Cedex 3, France; e-mail: Jean-Charles.Guery@inserm.fr.

![Figure 4. The persistence of donor-derived DCs in lymph nodes is impaired in the presence of host Ly49D+ NK cells. (A,B) B6 CD8−/− mice, treated or not with anti-NK1.1 PK136 mAb, were injected with CFSElow-labeled syngeneic B6 DCs and CFSEhigh-labeled allogeneic BALB/c DCs. Draining lymph nodes were harvested at indicated times after immunization and analyzed for the presence of CFSE-labeled cells. Cells were gated on CD11cpos, MHC IIhigh, and CFSEpos cells. (A) Representative CFSE profiles of injected DCs obtained at 48 hours from untreated or PK136-treated mice. (A,B) Numbers in dot plots and histograms indicate the percentages of allogeneic BALB/c CFSEhigh cells normalized to control syngeneic B6 CFSElow cells [(%BALB/c DCs:%B6 DCs) × 100]. (C) B6 CD8−/− mice were treated, or not, with anti-NK1.1 PK136 mAb or anti-Ly49D 4E5 mAb, and injected as in panel A with a mixture of CFSElow-B6 DCs and CFSEhigh-BALB/c DCs. Forty-eight hours after immunization, the presence of CFSE-positive cells was analyzed in the draining lymph nodes, and the percentage of CFSEhigh BALB/c cells among control CFSElow B6 cells was determined. Data are mean plus or minus SEM of 3 or 4 mice per group. (D) B6 CD8−/− mice treated, or not, with PK136 were injected with 1 or 2 × 106 CMTMR-labeled BALB/c DCs. Forty-eight hours after immunization, draining lymph nodes were harvested, prepared and stained with anti-B220 and anti-PNAd mAbs (blue). Lymph nodes sections were then analyzed by confocal microscopy, and the numbers of red DCs per lymph node were evaluated. Data are mean plus or minus SEM of 4 lymph nodes per group (2 mice/group). (E,F) B6 CD8−/− mice were treated or not with PK136 as indicated before immunization with DCs. (E) Mice were immunized with CMTMR-labeled BALB/c DCs (red). Frozen sections of lymph nodes at 48 hours were stained with goat anti-NKp46 antibodies and then donkey anti–goat Alexa488 antibodies. Numbers of NK cells and BALB/c DCs per section were counted. Data are mean plus or minus SEM of 4 to 6 lymph nodes per group. In panel F, mice were injected with CFSE-labeled BALB/c DCs (green) and CMTMR-labeled B6 DCs (red). Draining lymph nodes were removed at indicated times after immunization to evaluate the kinetics of allogeneic DC appearance. Lymph node sections were then stained with anti-B220 and anti-PNAd mAbs (blue). Representative tissue sections from untreated or PK136-treated mouse lymph nodes harvested at 48 hours after injection are depicted. Histograms indicate the percentage of CFSE BALB/c cells among control CMTMR B6 cells. Data are mean plus or minus SEM of individual lymph nodes (2 or 3 mice per group). *P < .05. **P < .01. N.S. indicates not significant. Data are representative of at least 2 or 3 experiments performed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/3/10.1182_blood-2007-10-120089/4/m_zh80160822020004.jpeg?Expires=1769192550&Signature=ofwR96ovveNjbl9hW4xpUcn1DaFatKo9vUFBHwK9kMdSZA5iBM5XbIVHj0v1t1zNcRTK19LuoqfD4ooA4L0RiB69YN2LIVDQDT~ruJvaI1iF-gkK~EaH8lwu5h8T12Qae4DRxyJSItzjv-bq3oQU2X497ybGIXJsMx27mwp6V~X2Ym3adEsNSlF0MuiNbE~X~aGg58FFUypMJuvCqFLQFu50QdpbhUySc0NzvxWkwBEFn9BX1v0ArCOn8j92XKV8B~Hl7GfPcKPlUBZPgTW-wn2MpQWDZCR7ucIQ-ewWKF5BLnSvWGT6mSVBH-4a4v1gFjOCQEStQXDXBaGpRPXlSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. The persistence of donor-derived DCs in lymph nodes is impaired in the presence of host Ly49D+ NK cells. (A,B) B6 CD8−/− mice, treated or not with anti-NK1.1 PK136 mAb, were injected with CFSElow-labeled syngeneic B6 DCs and CFSEhigh-labeled allogeneic BALB/c DCs. Draining lymph nodes were harvested at indicated times after immunization and analyzed for the presence of CFSE-labeled cells. Cells were gated on CD11cpos, MHC IIhigh, and CFSEpos cells. (A) Representative CFSE profiles of injected DCs obtained at 48 hours from untreated or PK136-treated mice. (A,B) Numbers in dot plots and histograms indicate the percentages of allogeneic BALB/c CFSEhigh cells normalized to control syngeneic B6 CFSElow cells [(%BALB/c DCs:%B6 DCs) × 100]. (C) B6 CD8−/− mice were treated, or not, with anti-NK1.1 PK136 mAb or anti-Ly49D 4E5 mAb, and injected as in panel A with a mixture of CFSElow-B6 DCs and CFSEhigh-BALB/c DCs. Forty-eight hours after immunization, the presence of CFSE-positive cells was analyzed in the draining lymph nodes, and the percentage of CFSEhigh BALB/c cells among control CFSElow B6 cells was determined. Data are mean plus or minus SEM of 3 or 4 mice per group. (D) B6 CD8−/− mice treated, or not, with PK136 were injected with 1 or 2 × 106 CMTMR-labeled BALB/c DCs. Forty-eight hours after immunization, draining lymph nodes were harvested, prepared and stained with anti-B220 and anti-PNAd mAbs (blue). Lymph nodes sections were then analyzed by confocal microscopy, and the numbers of red DCs per lymph node were evaluated. Data are mean plus or minus SEM of 4 lymph nodes per group (2 mice/group). (E,F) B6 CD8−/− mice were treated or not with PK136 as indicated before immunization with DCs. (E) Mice were immunized with CMTMR-labeled BALB/c DCs (red). Frozen sections of lymph nodes at 48 hours were stained with goat anti-NKp46 antibodies and then donkey anti–goat Alexa488 antibodies. Numbers of NK cells and BALB/c DCs per section were counted. Data are mean plus or minus SEM of 4 to 6 lymph nodes per group. In panel F, mice were injected with CFSE-labeled BALB/c DCs (green) and CMTMR-labeled B6 DCs (red). Draining lymph nodes were removed at indicated times after immunization to evaluate the kinetics of allogeneic DC appearance. Lymph node sections were then stained with anti-B220 and anti-PNAd mAbs (blue). Representative tissue sections from untreated or PK136-treated mouse lymph nodes harvested at 48 hours after injection are depicted. Histograms indicate the percentage of CFSE BALB/c cells among control CMTMR B6 cells. Data are mean plus or minus SEM of individual lymph nodes (2 or 3 mice per group). *P < .05. **P < .01. N.S. indicates not significant. Data are representative of at least 2 or 3 experiments performed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/3/10.1182_blood-2007-10-120089/4/m_zh80160822020004.jpeg?Expires=1769592169&Signature=G-BURo0YpnFVISzZFd~t~q~0aDC3u~bUR~DDPnpl0zhTY4J54oXQi04GL28DsA8pAnQpzxqRt-LUZJatHZmSxHcTAPnb4mxcLQzCivNoM9Dhq15yzZiErKTWGXk2bYZ-5H2KcdAh8GMKlcQNYTHtS4DKRxiJcjkHY3PoI-wZEHlV2lVhjzx0STuE75FpNQI2rUoZkpMm-mJc5WxP43Mea-AAwmp6kixqcbOL9Kv2j3~1BbZf3is1EUobTbGZX6IfVHYrZOv4ZE~an8hhvghPoGLRwGK2TKD2jgyWXQiz6tdORBQy9fMtiFCDl7dJ02jKGSy1c05CLnBj52nzX2hYBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)