Abstract
Background: Many patients with SCD require chronic transfusion therapy to manage the complications of their disease (eg stroke prevention); as a consequence, secondary iron overload may develop. Controlled data from patients with SCD receiving long-term iron chelation therapy, particularly renal function, are lacking. Cumulative 3.5-year safety and efficacy data are presented for adult and pediatric patients with SCD with transfusional iron overload treated with deferasirox (Exjade®) in a 4-year extension to a 1-year comparative study (109).
Methods: Study 109 demonstrated similar dose-dependent liver iron concentration (LIC) reductions with deferasirox and deferoxamine (DFO) in SCD patients with iron overload. Eligible patients entered a 4-year extension phase and received deferasirox only; dose adjustments were based on monthly serum ferritin (SF) levels and safety assessments (adverse events [AEs] and laboratory parameters). Patients with abnormal renal function were excluded.
Results: 132 patients (mean age ± SD of 19.1 ± 10.7 years) who were initially randomized in the core study to receive deferasirox are included in this analysis. The median duration of exposure to deferasirox was 37.4 months (3.1 years) at a mean dose of 18.4 ± 6.2 mg/kg/day. Mean iron intake over this period was 0.3 ± 0.1 mg/kg/day. 72 patients (54.5%) continue to receive deferasirox. Reasons for discontinuations include: AEs (n=11), consent withdrawal (n=24), lost to follow-up (n=9), unsatisfactory therapeutic effect (n=4), and other reasons (n=11). There was also one death reported post-liver transplantation, which was not considered by the investigator to be related to the study drug. The most frequent drug-related (investigator-assessed) AEs were nausea (n=20; 15.2%), diarrhea (n=14; 10.6%), vomiting (n=8; 6.1%) and abdominal pain (n=6; 4.5%). Nine patients (6.8%) had two consecutive increases in serum creatinine that were both >33% above baseline and above the upper limit of normal (ULN); however, there were no progressive increases. Five patients (3.8%) had an increase in alanine aminotransferase >10xULN on at least one visit; baseline levels were already >ULN in two patients.
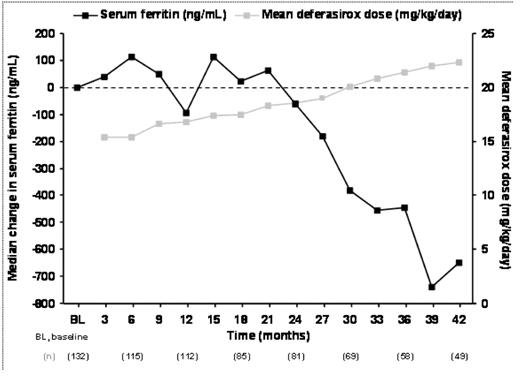
Mean daily dose of deferasirox increased from 15.4 ± 6.9 mg/kg/day at month 1 to 22.3 ± 7.3 mg/kg/day at month 42. Overall baseline median SF level was 3439 ng/mL (n=132), which decreased by 651 ng/mL (P=0.0533, Wilcoxon signed rank test; n=49) by month 42 (Figure 1). SF decreases were dose-dependent (data not shown).
Conclusions: Patients with SCD and transfusional iron overload receiving long-term deferasirox demonstrated continued reduction in their body iron burden (according to SF levels), without an exposure-associated increase in AE incidence, or evidence of progressive renal dysfunction.
Disclosures: Vichinsky:Novartis: Consultancy, Honoraria, Research Funding. Coates:Novartis: Honoraria, Research Funding, Speakers Bureau. Thompson:Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Bernaudin:Novartis: Investigator. Rodriguez:Novartis: Employment. Rojkjaer:Novartis: Employment. Heeney:Novartis: Research Funding. Off Label Use: ICL670 (deferasirox, Exjade) an oral iron chelator for the treatment of iron overload.
Author notes
Corresponding author