In a large study of children with acute ITP published in this issue of Blood, Neunert and colleagues find that irrespective of therapy aimed at raising the platelet count or the severity of thrombocytopenia, severe bleeding is rare.
Contrary to the exploits of Captain Nemo and his crew in Jules Verne's epic novel, there have been few hearty souls willing to venture below the surface of conventional childhood acute immune thrombocytopenic purpura (ITP) management and ask whether symptoms, platelet count, and intervention at diagnosis could predict bleeding severity and incidence in the next month. Accordingly, controversy still exists regarding management of ITP in these children. Specifically, should treatment be administered at all, for whom, and with which drugs? Drug treatment of ITP is often used to prevent serious bleeding, and the initial platelet count is assumed to be a surrogate for bleeding potential, especially for those who present with minimal bleeding. By most accounts, life-threatening hemorrhage, especially intracranial or central nervous system (CNS) bleeding, is quite rare (approximately 1 in 800 cases; 0.125%).1 But clinical anxiety often creeps in when there is mild mucosal bleeding, and the platelet count is firmly below sea level (less than or equal to 20 000 per mm3). This study by Neunert et al is the first to prospectively chronicle the severity of hemorrhage at diagnosis and in the next 4 weeks, as well as the relationship of clinical symptoms to the platelet count and treatment intervention.
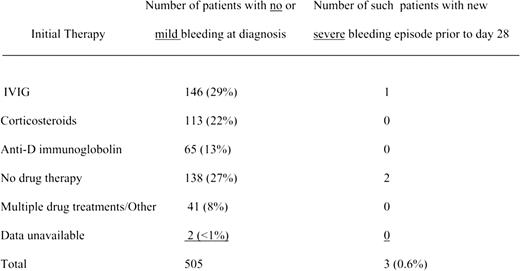
Eight hundred sixty-three of 1106 en-rolled patients were fully evaluable, and the vast majority had no or mild bleeding at diagnosis and a platelet count of less than or equal to 20 000 per mm3. Therapy aimed at raising the platelet count was left to provider discretion. The table illustrates the distribution of various treatments for those presenting with no to mild bleeding symptoms and the number of patients (n = 3) with serious bleeding within the first month after diganosis. Severe bleeding was defined as mucosal or CNS bleeding, requiring hospitalization and/or blood transfusions. All 3 patients had non-CNS bleeding. As expected, there was a significant difference between the platelet count and bleeding symptoms at diagnosis: mild bleeding equals 17 000/mm3, moderate equals 10 000/mm3, and severe equals 9000/mm3. However, there was no difference in subsequent severe bleeding in those with mild to moderate bleeding at diagnosis, whether or not treatment was given. Additionally, patients were more likely to receive treatment at diagnosis if their platelet count was low (range: 7000-12 000/mm3).
New severe hemorrhagic events during the first 28 days in patients with no or mild bleeding and a platelet count of less than or equal to 20 000 per mm3 at initial diagnosis. See the complete table in the article beginning on page 4003.
New severe hemorrhagic events during the first 28 days in patients with no or mild bleeding and a platelet count of less than or equal to 20 000 per mm3 at initial diagnosis. See the complete table in the article beginning on page 4003.
This investigation is important on several accounts. First, the prevalence of severe bleeding (including CNS) is similar to another large study (3%).2 Second, treatment did not statistically impact the development of severe bleeding by 4 weeks, even if the bleeding at diagnosis was moderate. Finally, similar to the findings from a prior study,3 treatment of major hemorrhage at diagnosis may not alter bleeding symptoms over the next several days. Words of caution regarding this study include a lack of validation of the bleeding score instrument and the inability to determine, by study design, the potential clinical benefits of judiciously raising the platelet count with drug intervention. As the authors suggest, serious bleeding in childhood acute ITP is quite rare, making design and completion of a definitive drug intervention trial that might show a difference in bleeding rates difficult. Accordingly, this author agrees with the investigators that subsequent studies in childhood ITP should be aimed at investigating quality of life (focusing on both treatment side effects/adverse events and the troublesome symptoms of bleeding without treatment), the cost of treatment, or identifying clinical or laboratory markers that may predict the development of severe bleeding during the first 28 days after diagnosis.
Even though there was a significant relationship at diagnosis between the platelet count and bleeding severity, the platelet count is not a good surrogate for treatment, as severe mucosal bleeding by itself would have dictated intervention. Also, even though most patients with a platelet count of less than or equal to 20 000/mm3 and mild to moderate bleeding symptoms did receive treatment at diagnosis (treatment: n=519; no treatment: n=157), there was no statistical difference in subsequent development of new severe bleeding at 4 weeks. In fact, only 6 patients experienced this complication (4 with treatment and 2 without). Currently, most clinicians still use a platelet count of less than or equal to 20 000 to institute treatment, regardless of the severity of bleeding symptoms. The data in this paper, however, would support allowing platelet counts to dive below this level and to treat based on clinical bleeding severity, thus avoiding costly and sometimes toxic treatment for patients who have only mild to moderate clinical bleeding at presentation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■