Drug resistance remains a critical problem in the treatment of patients with multiple myeloma. Recent studies have de-termined that Notch signaling plays a major role in bone marrow (BM) stroma-mediated protection of myeloma cells from de novo drug-induced apoptosis. Here, we investigated whether pharmacologic inhibition of Notch signaling could affect the viability of myeloma cells and their sensitivity to chemotherapy. Treatment with a γ-secretase inhibitor (GSI) alone induced apoptosis of myeloma cells via specific inhibition of Notch signaling. At concentrations toxic for myeloma cell lines and primary myeloma cells, GSI did not affect normal BM or peripheral blood mononuclear cells. Treatment with GSI prevented BM stroma-mediated protection of myeloma cells from drug-induced apoptosis. The cytotoxic effect of GSI was mediated via Hes-1 and up-regulation of the proapoptotic protein Noxa. In vivo experiments using xenograft and SCID-hu models of multiple myeloma demonstrated substantial antitumor effect of GSI. In addition, GSI significantly improved the cytotoxicity of the chemotherapeutic drugs doxorubicin and melphalan. Thus, this study demonstrates that inhibition of Notch signaling prevents BM-mediated drug resistance and sensitizes myeloma cells to chemotherapy. This may represent a promising approach for therapeutic intervention in multiple myeloma.
Introduction
Multiple myeloma (MM) is a hematologic malignancy resulting from a clonal proliferation of plasma cells in the bone marrow (BM).1 Although there have been major advances in the treatment of MM in recent years, it remains incurable mostly because of the development of drug resistance. Cell adhesion–mediated drug resistance represents an intrinsic resistance mechanism used by malignant cells that links cell adhesion to stroma or extracellular matrix with decreased sensitivity to chemotherapeutic drugs.2,3 Drug resistance to a wide variety of cytotoxic agents mediated by myeloma cell adhesion to BM stromal cells (BMS) or the extracellular matrix component fibronectin has been observed for several MM cell lines and primary patient specimens.2,4,–6
Notch signaling influences multiple processes that govern normal morphogenesis, programmed cell death, and cellular proliferation. It is initiated by binding of the extracellular domain of Notch to a Notch ligand. At present, 2 Notch ligand families, Delta and Jagged, have been described.7 The Notch receptors include 4 family members (Notch-1 to -4). Each member is a large single heterodimeric protein comprised of extracellular, transmembrane, and intracellular (ICN) subunits. Multiple lines of evidence have converged on a model in which the ICN translocates to the nucleus in a ligand-dependent fashion.8 Ligand-receptor interactions initiate 2 subsequent proteolytic cleavages within the transmembrane subunit, the first of which is mediated by ADAM metalloprotease. Subsequently, a second cleavage within the transmembrane domain releases the ICN. This cleavage requires γ-secretase activity of presenilin, a protein of a multisubunit complex that processes a number of transmembrane proteins in addition to Notch. ICN translocates to the nucleus where it interacts with the transcriptional repressor CSL, also known as CBF-1 (RBP-J). In the absence of the ICN, CSL/CBF-1 acts as a transcriptional repressor because of its ability to bind the transcriptional corepressor and histone deacetylase-1. Binding of the ICN displaces corepressor complexes and recruits coactivators, such as p300, PCAF, and GCN5, thus turning CSL/CBF-1 into a transcriptional activator.9,10 The targets of ICN/CSL signals in mammals include genes of the Hairy/Enhancer of Split (Hes) family, which encode bHLH-type trans-cription factors, HERP, HEY, as well as cyclins D1 and A, p21, and NF-κB.11,,,–15
There is ample evidence linking Notch and cancer. Overexpression of wild-type Notch receptors, ligands, and targets is observed in a growing number of solid tumors.16,,,–20 Similar defects were found in hematologic malignancies, including Hodgkin and non-Hodgkin lymphomas, subsets of acute myeloid leukemias, and B-cell chronic lymphoid leukemia.21,–23 Inhibition of Notch expression by antisense retrovirus or pharmacologic block of γ-secretase activity has striking antineoplastic effect in Notch-expressing transformed cells in vitro and in xenograft models.18,24,–26
These data suggest that interruption of Notch signaling may be a promising therapeutic strategy. Recently, we and others independently described the phenomenon of Notch activation in MM.27,28 In another study, activation of Notch signaling in MM cells resulted in inhibition of apoptosis.29 Overexpression of Notch ligand Jagged 2 was observed in MM cells. This overexpression resulted in secretion of interleukin-6 (IL-6), vascular endothelial growth factor, and insulin-like growth factor.30 These facts indicate an important role of Notch signaling in survival and growth of MM. Although Notch ligands can be detected on MM cells, they are most abundantly expressed by BMS and BM macrophages.27,31,–33 This in turn results in Notch activation and growth arrest resulting from up-regulation of p21 and decreased sensitivity of MM cells to chemotherapeutics.27 These data suggest that, in the context of tumor-stroma interaction, Notch signaling modulates the sensitivity of MM cells to chemotherapeutic drugs.
In this study, we investigated the effect of pharmacologic inhibition of Notch signaling on MM cell survival and chemosensitivity. We show that the specific block of Notch signaling with a γ-secretase inhibitor (GSI) results in MM cell apoptosis and also enhanced sensitivity to chemotherapeutics in vitro and in vivo. These data suggest that the addition of GSI may substantially improve the response of MM cells to standard chemotherapy.
Methods
Cell cultures and reagents
Human MM NCI-H929, U266, MM1S, and RPMI-8226 cell lines were cultured as described previously.27 BMS cultures were established from BM aspirates of healthy donors and grown in α-MEM medium as described previously.27 Blood samples were collected from healthy volunteers, and informed consent was obtained in accordance with the Declaration of Helsinki. Mononuclear cells (MNCs) were isolated by Ficoll gradient centrifugation and then cultured in α-MEM medium supplemented with 10% FBS. Melphalan and cycloheximide were purchased from Sigma-Aldrich (St Louis, MO), doxorubicin from Bedford Laboratories (Bedford, OH), and GSI (γ-secretase inhibitor XII) from Calbiochem (San Diego, CA).
Animals
NOD/SCID (NOD/MrkBomTac-Prkdcscid) and SCID-beige (C.B-Igh-1b/GbmsTac-Prkdcscid-Lystbg N7) mice were purchased from Taconic (Germantown, NY) and kept in pathogen-free conditions in the animal facility of the H. Lee Moffitt Cancer Center. All experimental procedures were performed in accordance with the guidelines of the University of South Florida Institutional Animal Care and Use Committee.
Xenograft mouse model
Six- to 8-week-old SCID/NOD mice were inoculated subcutaneously in the right flank with either 10 × 106 8226 cells or 5 × 106 H929 cells in 100 μL phosphate-buffered saline (PBS). In approximately 3 to 4 weeks, when the tumor became measurable, mice were assigned to one of the following 4 groups: control group, GSI or doxorubicin treated groups, or a group treated with the combination of both. GSI was administered daily for 14 days intraperitoneally at a dose of 5 mg/kg per day. Doxorubicin was injected intraperitoneally on the first day of the treatment at a dose of 1.5 mg/kg for the RPMI-8226 model and 0.75 mg/kg for the H929 model, and then was administered twice more at 4-day intervals. Tumor size was constantly monitored during and after treatment.
SCID-hu mouse model
The SCID-hu model was established as described previously.34 Briefly, human fetal tissues of 20 to 23 weeks of gestation were obtained from Advance Bioscience Resource (Alameda, CA) in compliance with the regulations issued by the state and federal governments. Fragments of human fetal bones (humerus, femur, or tibia) were implanted subcutaneously in 6-week-old female SCID-beige mice as described previously.35 Six weeks after implantation, 5 × 104 8226 myeloma cells in 50 μL PBS were injected directly into each implanted bone. Treatment with GSI, melphalan, doxorubicin, or combinations began approximately 4 weeks after tumor inoculation. All drugs were administered intraperitoneally. Melphalan was given twice with a 4-day interval at a dose of 1.5 mg/kg. GSI and doxorubicin were given as described for xenograft models. Mice in the control group received PBS. Blood (50–100 μL) was drawn from a submandibular vein and the level of free lambda light chain of human immunoglobulin was measured by an enzyme-linked immunosorbent assay (Bethyl, Montgomery, TX).
Measurement of GSI in serum
Serum samples were analyzed with a mass spectrometer in the selected ion monitoring mode set to obtain peaks at a mass to charge ratio (m/z) of 363 (M + H). The chromatographic separation of GSI was performed with a CN column using methanol and 0.1% formic acid mobile phases using an Agilent 1100 liquid chromatography/mass spectrometry system (Agilent Technologies, Palo Alto, CA). Chromatographic peaks were identified and quantified using Agilent Chemstation Software.
Isolation of primary myeloma cells
After obtaining informed consent from MM patients (approved by the University of South Florida Institutional Review Board), CD138+ myeloma cells were isolated from BM samples using CD138 MicroBeads and the MidiMACS magnetic cell separator according to the manufacturer's protocol (Miltenyi Biotec, Auburn, CA).
Colony formation assay
Human CD34+ hematopoietic progenitor cells were isolated from the BM of healthy donors using the CD34 progenitor cell isolation kit and magnetic beads (Miltenyi Biotec). CD34+ cells were cultured on monolayer of BMS for 24 hours. After that time, cells were collected, plated in duplicate into 6-well plates (5000 cells per well) in 1.1 mL of methylcellulose medium MethoCult GF H4434 (Stem Cell Technologies, Vancouver, BC) and treated with either GSI or vehicle control (dimethyl sulfoxide, DMSO). Colonies were scored on day 12.
Gene-expression array
H929 cells were treated with either DMSO or 6 μM GSI for 12 hours. The expression of genes involved in apoptosis was evaluated using Oligo GEArray Human Apoptosis Microarray (OHS-012, SuperArray Bioscience, Frederick, MD) according to the manufacturer's instructions. The signals were recorded using X-ray film and the chemiluminescent detection method. Data were analyzed using GEArray Expression Analysis software (SuperArray Bioscience).
Quantitative real-time polymerase chain reaction
MM cell lines and primary cells were kept in suspension or cultured on BMS, with or without GSI, for the period of time indicated. RNA was extracted and real-time polymerase chain reaction (PCR) was performed as described earlier27 on the ABI Prism 7900HT instrument using reagents from Applied Biosystems (Foster City, CA). The expression of specific genes was normalized to the expression of the endogenous control gene 18S.
MTT assay
Cells were treated with various concentrations of GSI for 48 hours. 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) dye was added for the last 4 hours of incubation. Insoluble formazan complexes were solubilized with DMSO and absorbance was measured at 540 nm using a Benchmark Plus microplate spectrophotometer (Bio-Rad, Hercules, CA). Each experimental condition was done in triplicate and repeated at least once.
Flow cytometry
Apoptosis of myeloma cells was detected by annexin V-PE/7-AAD staining as described earlier.27 A total of 10,000 events were acquired and analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and CellQuest software (BD Biosciences). Apoptosis of doxorubicin-treated cells was evaluated by annexin V-FITC/DAPI staining using LSRII (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR). Activation of Bak and Bax were determined by flow cytometry as described previously using specific antibodies that recognize only conformationally active forms of these proteins (anti-Bax [clone 3, BD Biosciences] or anti-Bak [Ab-1, Calbiochem]).36
Plasmids and cell transfection
Vector pIRES2-AcGFP1 was purchased from Clontech (Mountain View, CA). Vector pCMV-SPORT6ccdB containing human Hes-1 gene cDNA was obtained from Open Biosystems (catalog no. MHS1010-7429780, Huntsville, AL). Hes-1 was digested from cDNA clone with EcoR I and BseR I restriction endonucleases (New England Biolabs, Ipswich, MA), gel purified, and cloned into pIRES2-AcGFP1 vector. The presence of the insert was confirmed by sequencing. A Noxa siRNA pool or siCONTROL nontargeting siRNA pool was purchased from Dharmacon (Lafayette, CO). Vectors and siRNA were introduced into myeloma cells by electroporation using the Amaxa nucleofector device (Amaxa, Gaithersburg, MD). Nucleofector solutions V and R (Amaxa) and programs A-20 and T-16 were used for 8226 and U266 cells, respectively.
Luciferase assay
Cells stably transfected with the CBF-1 responsive element (p6xCBF1-IL-2-Luc), or control (IL-2-Luc) vector H929 cells were described in detail previously.27 H929 cells were cultured for 48 hours with or without GSI. After that time, cells were collected and the luciferase assay was performed as described27 using the Luciferase assay system kit (Promega, Madison, WI). Luciferase activity was normalized per 1 μg of protein.
Western blotting and immunoprecipitation
Western blotting and immunoprecipitation were performed as described previously.27,37 The antibodies against the following proteins were used: mcl-1, p53, Delta (C20), β-actin (Santa Cruz, Santa Cruz, CA); bcl-xL (BD Biosciences); Jagged-1, Jagged-2, bad, bcl-2, Puma, and Akt (Cell Signaling Technology, Danvers, MA); and Noxa (clone 114C307, Calbiochem).
Results
Effect of a γ-secretase inhibitor on Notch signaling and viability of myeloma cells
Our previous studies indicated that BMS protect MM cells from drug-induced apoptosis and that Notch signaling is among the leading mediators of this effect.2,27 Activation of Notch is mediated via the γ-secretase activity of the presenilin-containing macromolecular complex. We hypothesized that treatment of MM cells with a GSI would overcome BMS-mediated de novo drug resistance of MM cells.
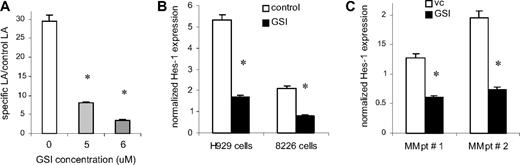
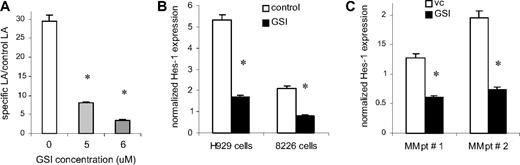
Experiments were performed to examine the ability of the GSI-XII (GSI) to specifically inhibit Notch signaling. H929 cells transfected with the CBF-1 reporter or control luciferase constructs were incubated on BMS monolayers established from 3 different normal donors. Cells were treated with GSI for 48 hours followed by measurement of luciferase activity. Treatment of H929 cells with 5 to 6 μM GSI resulted in a significant block of Notch activity (3.7- to 8.4-fold, P = .001; Figure 1A). It was possible that the GSI could nonspecifically shut down transcription. To determine whether the GSI effect on transcription was nonspecific, MM cells were transfected with the pGL3-promoter plasmid (Promega) containing luciferase under the SV40 promoter, and were treated with GSI for 48 hours. GSI did not affect the level of luciferase expression (data not shown). To confirm the specificity of the Notch inhibition further, we evaluated the effect of GSI on the expression of the Notch specific target gene Hes-1. Two MM cell lines (H929 and RPMI 8226) were cultured on BMS with or without GSI. The addition of GSI significantly down-regulated expression of the Hes-1 gene in both MM cell lines (Figure 1B). GSI was able to significantly decrease Hes-1 expression in primary CD138+ MM cells isolated from the BM of 2 patients with MM (Figure 1C). These data indicate that GSI inhibited Notch signaling in MM cells.
Treatment with GSI blocks Notch pathway activation in MM cells. (A) H929 cells stably transfected with the CBF-1 reporter or control vector were cultured on monolayer of BMS for 48 hours in the presence of vehicle control (DMSO, vc) or GSI. After that time, MM cells were collected and lysed using the reporter lysis buffer (Promega). The luciferase activity (LA) was normalized to 1 μg of total protein. Data are presented as fold increase of the specific LA (H929 cells transfected with p6 × CBF1-pKA9 plasmid) over control LA activity (cells transfected with pKA9 plasmid). Experiments were repeated on BMS from 3 different donors with similar results. (B,C) Myeloma H929 and 8226 cell lines (B) or primary CD138+ MM cells isolated from the BM of patients with MM (C) were cultured either in the presence of DMSO (vehicle control, vc) or GSI for 4 hours and 16 hours, respectively. The level of Hes-1 expression was determined in triplicate by real-time PCR and was normalized to the expression of housekeeping gene 18S (*P < .05, statistically significant difference in 2-tailed t test). Error bars represent SD.
Treatment with GSI blocks Notch pathway activation in MM cells. (A) H929 cells stably transfected with the CBF-1 reporter or control vector were cultured on monolayer of BMS for 48 hours in the presence of vehicle control (DMSO, vc) or GSI. After that time, MM cells were collected and lysed using the reporter lysis buffer (Promega). The luciferase activity (LA) was normalized to 1 μg of total protein. Data are presented as fold increase of the specific LA (H929 cells transfected with p6 × CBF1-pKA9 plasmid) over control LA activity (cells transfected with pKA9 plasmid). Experiments were repeated on BMS from 3 different donors with similar results. (B,C) Myeloma H929 and 8226 cell lines (B) or primary CD138+ MM cells isolated from the BM of patients with MM (C) were cultured either in the presence of DMSO (vehicle control, vc) or GSI for 4 hours and 16 hours, respectively. The level of Hes-1 expression was determined in triplicate by real-time PCR and was normalized to the expression of housekeeping gene 18S (*P < .05, statistically significant difference in 2-tailed t test). Error bars represent SD.
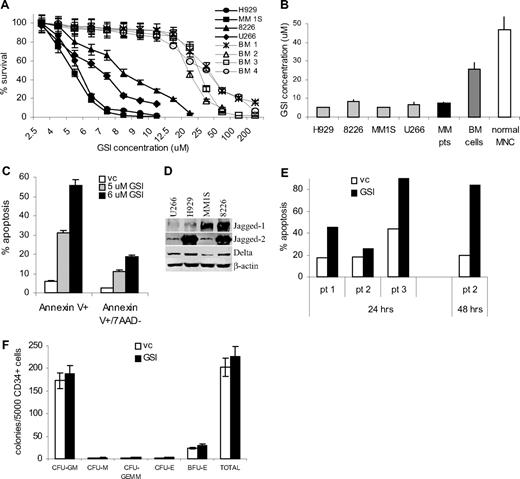
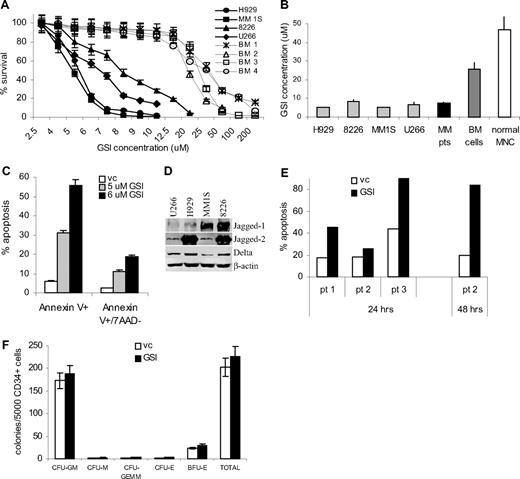
To investigate the antiproliferative effect of GSI on MM cells, we measured cellular viability using the MTT assay in different myeloma cell lines and primary MM cells isolated from BM of patients with MM. Peripheral blood MNCs and BM cells isolated from healthy donors were used as a control. GSI exerted significantly more toxic effect on myeloma cells compared with normal BM cells (Figure 2A,B). The IC50 was established for each cell line and primary cells. The effect of GSI on primary CD138+ MM cells was similar to its effect on MM cell lines. GSI was significantly less toxic to normal BM cells (IC50 25.5 ± 4.8 μM; range, 18.8–30.4 μM, P = .006) and MNCs (IC50 47 ± 28 μM; range, 25–83.5 μM, P = .026; Figure 2A,B). Importantly, the IC50 for MM cell lines and primary cells was at the level where inhibition of Notch activity was detected (Figure 1A).
Treatment with GSI induces MM cell death. (A,B) H929, U266, MM1S, or RPMI 8226 cells (5 × 104), 105 myeloma cells obtained from BM aspirates of 4 MM patients, or 105 BM cells from 4 healthy donors or PB MNCs obtained from 5 donors were plated per well of 96-well plates and treated with GSI for 48 hours followed by the MTT test. (A) Cell viability curves for MM cell lines and normal BM cells are shown. (B) IC50 values were calculated for all types of cells studied. The differences between the values in MM cell lines and primary MM cells and control BM or PB MNCs were statistically significant in 2-tailed t test (P < .002). (C) H929 MM cell line or (E) primary CD138+ myeloma cells were treated with GSI or vehicle control for 24 hours. Proportion of apoptotic cells was analyzed by annexin V-PE/7-AAD staining using FACSCalibur flow cytometer. (D) The expression of Notch ligands on MM cells was analyzed by Western blotting. Membranes were reprobed with β-actin antibody to confirm protein loading. (F) CD34+ cells were isolated from the BM of healthy donors and cultured on the monolayer of BMS for 24 hours. After that time, CD34+ cells were collected and plated in semi-solid media according to the manufacturer's protocol. A total of 5000 CD34+ cells were plated per well and cultured either in the presence of DMSO (control) or 8 μM GSI. Experiments were performed in duplicate and repeated once. Colonies were scored using an inverted microscope. The numbers of colonies per 5 × 103 CD34+ cells are shown. CFU indicates colony-forming units; GM, granulocyte/macrophage; M, macrophage; E, erythrocyte; GEMM, mixed; and BFU-E, burst forming units erythroid. Error bars represent SD.
Treatment with GSI induces MM cell death. (A,B) H929, U266, MM1S, or RPMI 8226 cells (5 × 104), 105 myeloma cells obtained from BM aspirates of 4 MM patients, or 105 BM cells from 4 healthy donors or PB MNCs obtained from 5 donors were plated per well of 96-well plates and treated with GSI for 48 hours followed by the MTT test. (A) Cell viability curves for MM cell lines and normal BM cells are shown. (B) IC50 values were calculated for all types of cells studied. The differences between the values in MM cell lines and primary MM cells and control BM or PB MNCs were statistically significant in 2-tailed t test (P < .002). (C) H929 MM cell line or (E) primary CD138+ myeloma cells were treated with GSI or vehicle control for 24 hours. Proportion of apoptotic cells was analyzed by annexin V-PE/7-AAD staining using FACSCalibur flow cytometer. (D) The expression of Notch ligands on MM cells was analyzed by Western blotting. Membranes were reprobed with β-actin antibody to confirm protein loading. (F) CD34+ cells were isolated from the BM of healthy donors and cultured on the monolayer of BMS for 24 hours. After that time, CD34+ cells were collected and plated in semi-solid media according to the manufacturer's protocol. A total of 5000 CD34+ cells were plated per well and cultured either in the presence of DMSO (control) or 8 μM GSI. Experiments were performed in duplicate and repeated once. Colonies were scored using an inverted microscope. The numbers of colonies per 5 × 103 CD34+ cells are shown. CFU indicates colony-forming units; GM, granulocyte/macrophage; M, macrophage; E, erythrocyte; GEMM, mixed; and BFU-E, burst forming units erythroid. Error bars represent SD.
Next, we asked whether the decreased viability of MM cells after treatment with GSI was due to induction of apoptosis. Four MM cell lines or primary cells isolated from 3 patients were treated with GSI for 24 hours followed by annexin V/7-AAD staining. GSI induced significant apoptosis of all MM cell lines tested (Figure 2C and data not shown). Expression of Notch ligands Jagged-1, -2, and Delta by MM cells (Figure 2D) suggested that in suspension Notch pathway was activated by MM cells interacting with each other. GSI also induced apoptosis of primary MM cells (Figure 2E).
BM toxicity could be a major limiting factor for the use of GSI. We therefore evaluated the effect of GSI on BM progenitor cells at doses that are toxic for MM cells (5–8 μM). CD34+ hematopoietic progenitor cells were isolated from normal BM and were cultured on monolayer of BMS for 24 hours. After that, CD34+ cells were collected and plated in methylcellulose media supplemented with growth factors supporting myeloid and erythroid colonies in the presence of 5 to 8 μM GSI. Colonies were scored after 12 days of culture. At all selected doses, GSI did not affect the colony-forming ability of CD34+ progenitor cells (Figure 2F). Thus, taken together, these results demonstrate that GSI inhibits Notch signaling and induces apoptosis of MM cells without affecting normal hematopoietic progenitor cells.
GSI enhances the effect of chemotherapy on MM cells in vivo
We previously demonstrated that BMS decreased the sensitivity of MM cells to chemotherapeutic agents.2 Here, we investigated whether GSI could increase MM cell drug response in the presence of BMS. The antimyeloma effect of both GSI and doxorubicin at selected doses was rather modest. However, cytotoxicity was significantly enhanced by the combination of these 2 drugs (Figure 3A). Similarly, GSI prevented the BMS protective effect when cells were treated with mitoxantrone (data not shown).
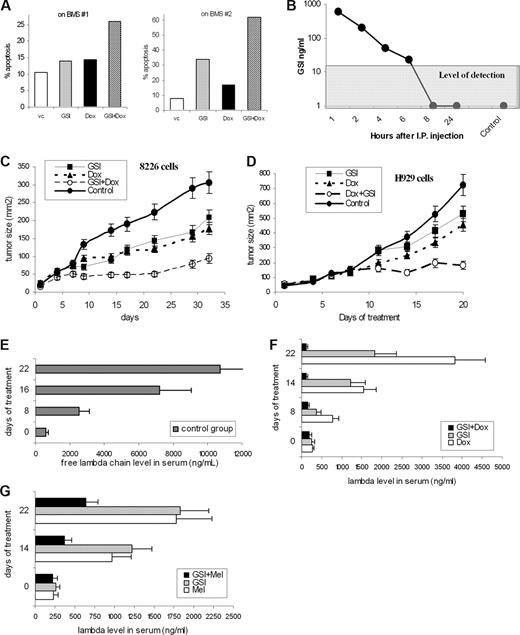
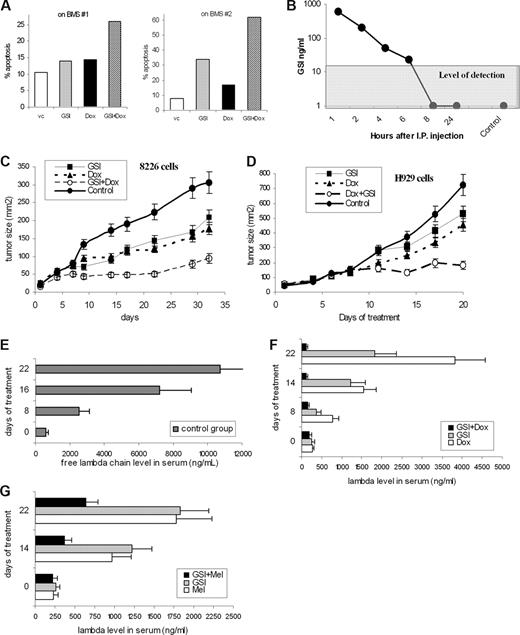
Combined effect of GSI and chemotherapeutics on MM cells. (A) H929 cells were cultured overnight on a BMS monolayer established from 2 different donors with or without 5 μM GSI. Doxorubicin (Dox, 0.25 μM) was then added to the cultures where indicated and cells were cultured for an additional 24 hours. Apoptosis of myeloma cells was evaluated by annexin-FITC/DAPI staining using an LSR II flow cytometer. (B) GSI (5 mg/kg) was injected intraperitoneally into SCID/NOD mice (2 mice per time point). Blood was collected at different time points, and the concentration of GSI was determined in sera as described in “Measurement of GST in serum.” Control indicates mice not treated with the compound. (C,D) RPMI-8226 (C) or H929 (D) tumors were established subcutaneously in SCID/NOD mice. Mice were split into 4 groups (5 mice per group) with equal size tumors and treated with GSI, doxorubicin, or a combination thereof. Tumor growth was monitored. Four experiments with similar results were performed; 2 of them are shown. Mean plus or minus SD values are shown. (E-G) The SCID-hu model was established as described in “SCID-hu mouse model.” Tumor growth was monitored by measuring the level of human paraprotein in mouse sera. Approximately 4 weeks after tumor injection, mice were split into 4 groups (3–4 mice per group) with equal average level of free lambda light chain in the sera. Mice were treated with GSI, doxorubicin, melphalan, or a combination of GSI with chemotherapeutic drug (either doxorubicin or melphalan) for 14 days. Please note the different scale for control group (E) and treated groups of mice (F,G). Mice were killed 1 week after the end of the treatment. Mean plus or minus SD values are shown.
Combined effect of GSI and chemotherapeutics on MM cells. (A) H929 cells were cultured overnight on a BMS monolayer established from 2 different donors with or without 5 μM GSI. Doxorubicin (Dox, 0.25 μM) was then added to the cultures where indicated and cells were cultured for an additional 24 hours. Apoptosis of myeloma cells was evaluated by annexin-FITC/DAPI staining using an LSR II flow cytometer. (B) GSI (5 mg/kg) was injected intraperitoneally into SCID/NOD mice (2 mice per time point). Blood was collected at different time points, and the concentration of GSI was determined in sera as described in “Measurement of GST in serum.” Control indicates mice not treated with the compound. (C,D) RPMI-8226 (C) or H929 (D) tumors were established subcutaneously in SCID/NOD mice. Mice were split into 4 groups (5 mice per group) with equal size tumors and treated with GSI, doxorubicin, or a combination thereof. Tumor growth was monitored. Four experiments with similar results were performed; 2 of them are shown. Mean plus or minus SD values are shown. (E-G) The SCID-hu model was established as described in “SCID-hu mouse model.” Tumor growth was monitored by measuring the level of human paraprotein in mouse sera. Approximately 4 weeks after tumor injection, mice were split into 4 groups (3–4 mice per group) with equal average level of free lambda light chain in the sera. Mice were treated with GSI, doxorubicin, melphalan, or a combination of GSI with chemotherapeutic drug (either doxorubicin or melphalan) for 14 days. Please note the different scale for control group (E) and treated groups of mice (F,G). Mice were killed 1 week after the end of the treatment. Mean plus or minus SD values are shown.
To determine the effect of GSI in vivo, we first evaluated toxicity and serum kinetics of this compound. SCID/NOD mice were treated for 14 days with daily intraperitoneal injections of GSI at different concentrations. GSI at a dose of 5 mg/kg per day did not cause any visible signs of toxicity during treatment. To evaluate the kinetics of GSI after intraperitoneally injections, serum concentrations of GSI were measured at different time points. The level of detection in this experiment was 20 ng/mL. The GSI concentration in sera decreased rapidly after injection, and it was not detectable 8 hours after administration (Figure 3B). The concentration achieved in serum was approximately 3 to 4 μM, which was close to the effective GSI concentration used in vitro. Based on these data, we selected a GSI dose of 5 mg/kg per day.
To investigate the effect of GSI in vivo we used 2 different MM mouse models. First, a xenograft model used subcutaneous injection of MM 8226 or H929 cells into a flank of SCID/NOD mice. Because MM cells grow as a homogeneous solid tumor in this model, the Notch pathway is primarily activated by MM cells interacting with each other. When the tumor mass was palpable, mice were split into 4 groups and treated with GSI, doxorubicin, or a combination thereof. The antitumor effect of GSI or doxorubicin alone at selected doses was rather moderate (Figure 3C,D). However, the combination of these 2 drugs dramatically reduced growth of both 8226 and H929 tumors (Figure 3C,D).
One of the disadvantages of the xenograft model is that MM cells grow as a solid tumor, which is artificial for MM. Therefore, to evaluate the effect of GSI and its combination with chemotherapeutics in the context of human BM, we used an SCID-hu model, where 8226 MM cells were injected directly into previously engrafted fragments of human bone. In this model, tumor growth was monitored by measuring the level of a specific human paraprotein in mouse serum. Mice were treated with GSI, melphalan or doxorubicin, or a combination of GSI with chemotherapeutics. Administration of each of these drugs alone decreased the level of free human lambda chain in serum (Figure 3E-G). Addition of GSI to either melphalan or doxorubicin dramatically enhanced the effect of chemotherapeutics (Figure 3F,G).
Proapoptotic effect of GSI on MM cells is mediated by Hes-1 down-regulation
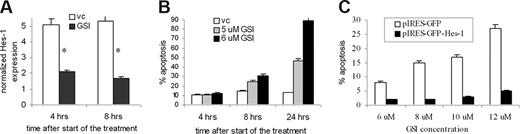
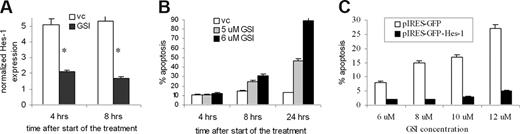
Because GSI may have a pleiotropic effect on MM cells, we studied whether the induction of apoptosis by GSI was indeed mediated by block of Notch signaling. First, we determined that inhibition of Notch signaling by GSI preceded the induction of apoptosis. H929 cells were treated with GSI, and the level of apoptosis and Notch signaling activation was measured at different time points. GSI dramatically reduced expression of the Notch specific downstream target Hes-1, as early as 4 hours after the start of treatment (Figure 4A). At that time, no induction of apoptosis was detected. A significant increase in apoptosis of H929 cells was detected after 8 hours of treatment (Figure 4B). Similar results were obtained for another MM cell line, RPMI-8226 (data not shown).
GSI effect on MM cells is mediated through Hes-1. (A,B) H929 cells were treated with GSI for 4, 8, or 24 hours. After that time, cells were collected and (A) the expression of Hes-1 gene was determined by real-time PCR as described in “Quantitative real-time polymerase chain reaction” and normalized to the expression of housekeeping gene 18S. * indicates statistically significant difference (P < .05) in 2-tailed t test. (B) In parallel, cells were stained with annexin V/7-AAD and the level of apoptosis was detected by flow cytometry. (C) Overexpression of Hes-1 abrogated GSI-induced apoptosis in MM cells. Human U266 MM cells were transfected with either pIRES2-AcGFP-Hes-1 or control empty pIRES2-AcGFP vector and treated with the indicated concentrations of GSI for 24 hours. Apoptosis was measured by flow cytometry in a gated GFP-positive population of cells by annexin V-PE/7-AAD staining using FACSCalibur. Error bars represent SD.
GSI effect on MM cells is mediated through Hes-1. (A,B) H929 cells were treated with GSI for 4, 8, or 24 hours. After that time, cells were collected and (A) the expression of Hes-1 gene was determined by real-time PCR as described in “Quantitative real-time polymerase chain reaction” and normalized to the expression of housekeeping gene 18S. * indicates statistically significant difference (P < .05) in 2-tailed t test. (B) In parallel, cells were stained with annexin V/7-AAD and the level of apoptosis was detected by flow cytometry. (C) Overexpression of Hes-1 abrogated GSI-induced apoptosis in MM cells. Human U266 MM cells were transfected with either pIRES2-AcGFP-Hes-1 or control empty pIRES2-AcGFP vector and treated with the indicated concentrations of GSI for 24 hours. Apoptosis was measured by flow cytometry in a gated GFP-positive population of cells by annexin V-PE/7-AAD staining using FACSCalibur. Error bars represent SD.
We have previously found that Notch activation leads to up-regulation of p21waf/kip and growth arrest of MM cells.27 We tested the hypothesis that GSI would reverse this effect. Treatment of MM cells with GSI for 48 hours did not result in increased cell proliferation or down-regulation of p21waf/kip (data not shown).
The Hes-1 transcription factor is one of the major targets of Notch. We investigated whether Hes-1 was directly involved in MM cell protection from drug-induced apoptosis. To test this hypothesis, Hes-1 was overexpressed in MM cells. U266 cells were transfected with the bi-cistronic vector pIRES-AcGFP-Hes-1 or control empty vector, and then exposed to GSI for 24 hours. Apoptosis of transfected (GFP positive) cells was measured by flow cytometry using annexin V-PE/7-AAD staining. Overexpression of Hes-1 abrogated GSI-induced apoptosis in MM cells (Figure 4C). These data confirm that GSI induces apoptosis in MM cells through inhibition of Notch signaling and indicate that Notch-induced protection of MM cells is mediated via Hes-1.
Mechanism of apoptosis induced by inhibition of Notch signaling
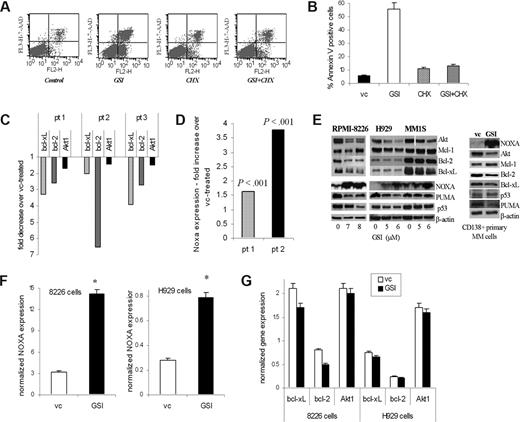
To determine whether GSI-induced apoptosis requires protein synthesis, MM H929 cells were pretreated with cycloheximide followed by exposure to GSI. Cycloheximide completely abrogated GSI-induced apoptosis of MM cells (Figure 5A,B), indicating that GSI-induced apoptosis is likely dependent on up-regulation of proapoptotic protein(s).
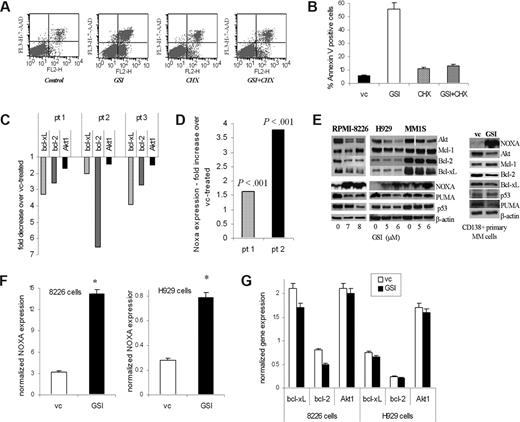
Downstream targets of GSI in MM cells. (A,B) Pretreatment with cycloheximide (CHX) abrogates the effect of GSI on MM cells. H929 cells were pretreated with 0.5 μg/mL CHX for 2 hours followed by treatment with 5 μM GSI for 24 hours. Apoptosis was measured by flow cytometry using annexin V/7-AAD staining as described in “Flow cytometry.” A typical flow cytometry dot-plot picture (A) and cumulative results from 3 experiments (B) are shown. (C,D) Primary CD138+ MM cells were treated in triplicate with GSI or DMSO (vehicle control, vc) for 18 hours. Cells were then collected and RNA was extracted. Expression of indicated genes was evaluated by real-time PCR and normalized to the expression of housekeeping gene 18S. Data are presented as a fold change in GSI treated cells compared with vehicle control-treated cells. In panel C, P < .05 for all genes evaluated. In panel D, actual P values are shown. (E) MM cell lines and primary MM cells were treated with GSI for 24 hours. After that time, cells were collected and the levels of indicated proteins were determined by Western blotting. As a loading control, membranes were reprobed with antibody against β-actin. MM1S cells were treated with 3 different GSI concentrations (4, 5, and 6 μM), and then all of them or only cells treated with the 2 highest doses were used for Western blotting. A portion of the gel (Akt) where cells were treated with 3 doses was repositioned to keep consistency with the 2 highest GSI concentrations used. The reposition is indicated by the vertical white line. (F,G) MM H929 or 8226 cell lines were treated with GSI or VC for 4 hours. Expression of Bcl-2 family and Akt genes (G) or Noxa (F) was evaluated by real-time PCR and normalized to the expression of endogenous control gene 18S. *Statistically significant difference (P < .05) in 2-tailed t test. Error bars represent SD.
Downstream targets of GSI in MM cells. (A,B) Pretreatment with cycloheximide (CHX) abrogates the effect of GSI on MM cells. H929 cells were pretreated with 0.5 μg/mL CHX for 2 hours followed by treatment with 5 μM GSI for 24 hours. Apoptosis was measured by flow cytometry using annexin V/7-AAD staining as described in “Flow cytometry.” A typical flow cytometry dot-plot picture (A) and cumulative results from 3 experiments (B) are shown. (C,D) Primary CD138+ MM cells were treated in triplicate with GSI or DMSO (vehicle control, vc) for 18 hours. Cells were then collected and RNA was extracted. Expression of indicated genes was evaluated by real-time PCR and normalized to the expression of housekeeping gene 18S. Data are presented as a fold change in GSI treated cells compared with vehicle control-treated cells. In panel C, P < .05 for all genes evaluated. In panel D, actual P values are shown. (E) MM cell lines and primary MM cells were treated with GSI for 24 hours. After that time, cells were collected and the levels of indicated proteins were determined by Western blotting. As a loading control, membranes were reprobed with antibody against β-actin. MM1S cells were treated with 3 different GSI concentrations (4, 5, and 6 μM), and then all of them or only cells treated with the 2 highest doses were used for Western blotting. A portion of the gel (Akt) where cells were treated with 3 doses was repositioned to keep consistency with the 2 highest GSI concentrations used. The reposition is indicated by the vertical white line. (F,G) MM H929 or 8226 cell lines were treated with GSI or VC for 4 hours. Expression of Bcl-2 family and Akt genes (G) or Noxa (F) was evaluated by real-time PCR and normalized to the expression of endogenous control gene 18S. *Statistically significant difference (P < .05) in 2-tailed t test. Error bars represent SD.
To identify the protein(s) responsible for the proapoptotic effect of GSI on MM cells, we used Oligo GEarray technology (SuperArray Bioscience). H929 cells were treated with GSI or vehicle control, and the expression of 112 key genes involved in apoptosis was evaluated. Expression of the 3 antiapoptotic genes Akt, Bcl-xL, and Bcl-2 was reproducibly down-regulated by GSI. In a separate set of experiments, we observed that GSI dramatically induced expression of the proapoptotic BH3-only protein Noxa. These results were validated by real-time PCR (data not shown). To verify the effect of GSI on primary MM cells, CD138+ cells isolated from the BM of patients with MM were treated with GSI. Inhibition of Notch signaling resulted in a decrease of the expression of Bcl-xL and Bcl-2 (P < .001) and a slight but significant decrease in the expression of Akt1 (P = .03) (Figure 5C). At the same time, expression of Noxa was dramatically up-regulated by GSI (Figure 5D).
Reduction in the protein level of Akt was found in all MM cell lines and primary MM cells tested, whereas the decrease in bcl-2 was much less evident (Figure 5E). The level of bcl-xL protein was down-regulated in MM cell lines 8226 and MM1S but only slightly decreased in primary MM cells. At the same time, the level of Noxa protein was significantly increased in all MM cell lines and primary MM cells tested (Figure 5E).
Although Noxa is a major component of p53-dependent apoptosis, its involvement in a p53-independent pathway has also been established.38 To clarify a potential role of p53 in GSI-mediated apoptosis of MM cells, we used MM cell lines with wild-type (H929) and mutant (8226) p53.39 GSI was able to induce apoptosis equally in both cell lines (data not shown), which suggest that GSI is likely to exert its effect in a p53-independent manner. Puma is another proapoptotic member of the BH3-only family with a similar mechanism of action as Noxa. However, in contrast to the effect on Noxa, GSI did not up-regulate the level of Puma in primary MM cells or MM cell lines (Figure 5E).
A dramatic increase in the expression of Noxa was detected as early as 4 hours after treatment with GSI (Figure 5F) and coincided with inhibition of Notch signaling (Figure 4A). In contrast, changes in the expression of Bcl-xL, Bcl-2, or Akt-1 were not evident at that time point (Figure 5G).
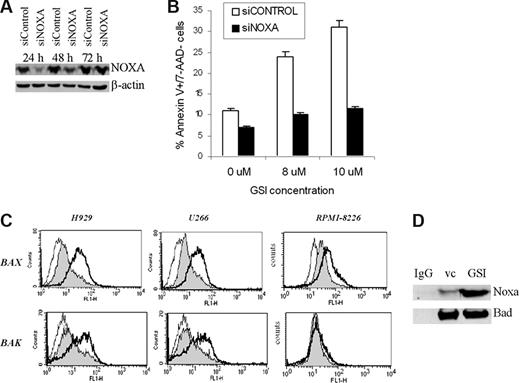
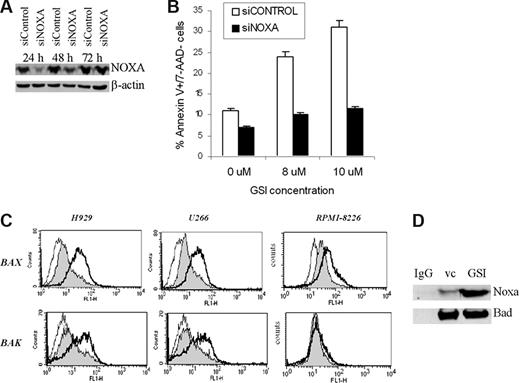
To clarify the role of Noxa in GSI-induced apoptosis, we knocked it down in 8226 cells using specific siRNA (Figure 6A); 8226 cells were treated with different concentrations of GSI and apoptosis was evaluated using annexin V/7AAD staining. A decrease of Noxa protein level prevented GSI-induced apoptosis of MM cells (Figure 6B).
GSI induces apoptosis of MM cells by up-regulation of Noxa. Introduction of Noxa siRNA into MM cells cancels GSI-induced apoptosis. Human 8226 cells were transfected with Noxa siRNA or siCONTROL nontargeting pool siRNA using Amaxa nucleofector device. (A) Cells were incubated for the indicated period of time, and the levels of Noxa were determined by Western blotting. As a loading control, the membrane was reprobed with antibody against β-actin. (B) Transfected cells were treated in triplicate with either vehicle control (DMSO) or the indicated concentrations of GSI. After 12 hours, cells were collected, and apoptosis was measured by annexin V/7-AAD staining using FACSCalibur flow cytometer. Mean plus or minus SD values are shown. The differences were statistically significant for all GSI concentrations (P < .05). (C) Bax and Bak were activated after Noxa up-regulation. MM cells were treated with vehicle control (filled histogram) or GSI (thick line) for 16 hours, then collected and fixed. Staining with antibodies against the conformationally active forms of Bak and Bax was performed as described in “Flow cytometry.” As a control, cells were stained with secondary antibody only (thin line). Cells were analyzed by flow cytometry, and typical histograms from 3 experiments are shown. (D) 8226 MM cells were treated with 7 μM GSI for 24 hours. After that time, cells were collected, lysed, and immunoprecipitated with an antibody against Bad or normal rabbit IgG. The membrane was probed with anti-Noxa and anti-Bad antibody. Three experiments yielded similar results.
GSI induces apoptosis of MM cells by up-regulation of Noxa. Introduction of Noxa siRNA into MM cells cancels GSI-induced apoptosis. Human 8226 cells were transfected with Noxa siRNA or siCONTROL nontargeting pool siRNA using Amaxa nucleofector device. (A) Cells were incubated for the indicated period of time, and the levels of Noxa were determined by Western blotting. As a loading control, the membrane was reprobed with antibody against β-actin. (B) Transfected cells were treated in triplicate with either vehicle control (DMSO) or the indicated concentrations of GSI. After 12 hours, cells were collected, and apoptosis was measured by annexin V/7-AAD staining using FACSCalibur flow cytometer. Mean plus or minus SD values are shown. The differences were statistically significant for all GSI concentrations (P < .05). (C) Bax and Bak were activated after Noxa up-regulation. MM cells were treated with vehicle control (filled histogram) or GSI (thick line) for 16 hours, then collected and fixed. Staining with antibodies against the conformationally active forms of Bak and Bax was performed as described in “Flow cytometry.” As a control, cells were stained with secondary antibody only (thin line). Cells were analyzed by flow cytometry, and typical histograms from 3 experiments are shown. (D) 8226 MM cells were treated with 7 μM GSI for 24 hours. After that time, cells were collected, lysed, and immunoprecipitated with an antibody against Bad or normal rabbit IgG. The membrane was probed with anti-Noxa and anti-Bad antibody. Three experiments yielded similar results.
It is known that Noxa exerts its proapoptotic effects through binding to the pro-survival protein Mcl-1. This results in dissociation and activation of Bax and Bak, followed by mitochondrial outer membrane permeabilization and induction of apoptosis. We investigated whether GSI affected Bak and Bax. According to gene expression array data, GSI did not affect the RNA level of these genes (data not shown). However, it induced proapoptotic conformational changes of these proteins, as was determined by flow cytometry (Figure 6C). GSI was able to activate Bax 1.7- to 2.7-fold in H929, U266, and RPMI-8226 cells. It substantially activated Bak in H929 and U266 cells (1.7- to 2.2-fold) and slightly (1.4 ± 0.3-fold) in RPMI-8226 cells (Figure 6C). Noxa can also induce apoptosis in association with Bad. To test whether GSI could induce a Noxa–Bad interaction, 8226 MM cells were treated with GSI and whole cell lysates were immunoprecipitated with an antibody against Bad. Membranes were probed with a anti-Noxa antibody. Binding of Noxa to Bad was substantially increased in GSI-treated cells compared with cells treated with vehicle control (Figure 6D). These data indicate that Bad can be one of the downstream targets for Noxa in mediating GSI-induced apoptosis.
Discussion
The results presented in this study demonstrate, for the first time, that the pharmacologic inhibition of Notch signaling may enhance the effect of chemotherapeutics in MM. MM cells reside primarily in the BM where they interact with components of the BM microenvironment, including BMS. Increasing evidence has supported the idea that the interaction of tumor cell with its microenvironment has a profound effect on growth, survival, and chemosensitivity of malignant cells. Previously, we and others have linked resistance of tumor cells to chemotherapy with their adhesion to BMS.2,3,40,41 Our data indicate that Notch signaling is among the major factors responsible for this phenomenon.27 We hypothesized that blocking Notch signaling would reverse the BMS-mediated de novo drug resistance and sensitize MM cells to chemotherapeutics.
Activation of Notch requires receptor cleavage by a multisubunit complex possessing γ-secretase activity. Therefore, to block Notch signaling, we used a γ-secretase inhibitor. It was selected after preliminary experiments had demonstrated specific activity against Notch, which was determined using an analysis of the CBF-1 reporter and the target Notch gene Hes-1. We have previously found that activation of Notch signaling lead to up-regulation of p21 and growth arrest of MM cells in the G0/G1 phase of the cell cycle.27 Our initial hypothesis was that GSI would block activation of the Notch pathway caused by MM cell interaction with either BMS or between MM cells (MM cells express both the Notch receptor and Notch ligand), reverse growth arrest of MM cells and remove Notch-mediated protection of these cells from drug-induced apoptosis. However, treatment of MM cells with GSI did not result in increased cell proliferation and down-regulation of p21 but rather rapidly induced apoptosis of MM cells. In this situation, the reversal of the effect of Notch signaling on p21 or cell cycle cannot be detected.
The majority of the downstream effects of Notch are mediated via the Hes family of transcriptional factors.11 Our experiments have demonstrated that inhibition of Hes-1 transcription preceded the induction of apoptosis in MM cells. Importantly, overexpression of Hes-1 abrogated the proapoptotic effect of GSI on MM cells. Taken together, these data strongly suggest that Hes-1 is the mediator of the cytotoxic effect of GSI and further confirm the specific effect of GSI on Notch signaling in MM cells. Cellular apoptosis is governed largely by the interaction between members of the Bcl-2 family, including pro-survival and proapoptotic proteins. The multidomain proteins Bak and Bax, when activated, permeabilize the outer mitochondria membrane and trigger the release of cytochrome c needed to activate caspases and induce apoptosis.42 The BH3-only proteins trigger apoptosis either by binding via their BH3 domain to antiapoptotic members of the Bcl-2 family, including Bcl-2, Bcl-xL, and Mcl-1 and displacing them from Bax and Bak (Noxa, Puma, Bad), or by inducing conformational changes and activation of Bax or Bak (Bid, Bim).43
Our study has demonstrated that GSI rapidly and dramatically up-regulated the proapoptotic protein Noxa in MM cell lines and primary MM cells. Most importantly, down-regulation of Noxa with siRNA completely prevented the induction of apoptosis by GSI. These data argue in favor of Noxa as the main target of GSI mediated inhibition of Notch signaling in MM cells. It appears that activation of Noxa by GSI is not restricted only to MM. In a recent study, the potential role of this protein in GSI-mediated induction of apoptosis in melanoma cells was shown.44 It is known that Noxa exerts its proapoptotic effects through its binding to the pro-survival protein Mcl-1, which results in dissociation and activation of Bax and Bak. Noxa binding to Mcl-1 may result in degradation of this protein (reviewed by Shibue and Taniguchi38 ). However, the loss of Mcl-1 expression does not necessarily induce apoptosis in each case, as revealed by gene-targeting and RNA interference studies.38 It is possible that, as was recently shown, Noxa may effectively induce apoptosis in association with Bad.45 Our experiments support this point of view because GSI substantially increase Noxa binding to Bad in 8226 cells.
Based on the results of our experiments, we can suggest a mechanism of GSI-induced apoptosis of MM cells. Activation of Notch, which takes place during the MM cell interaction with BMS, may result in the accumulation of Hes-1, which functions as a transcriptional repressor.46 Hes-1 may directly suppress Noxa expression. The analysis of the promoter region of Noxa demonstrated the presence of 2 consensus Hes-1 binding sites. However, there are no data yet available that would directly demonstrate repression of Noxa by Hes-1. Blocking of Notch signaling with GSI down-regulates Hes-1, which leads to an up-regulation of Noxa, displacement of Bax and Bak, and activation of apoptosis. GSI can easily overcome BMS induced activation of Notch, which results in reversal of BMS-mediated protection of MM from apoptosis. This makes MM cells more sensitive to the effects of chemotherapeutic drugs.
Our data using 2 different in vivo models demonstrate that GSI substantially enhanced the antitumor effect of 2 different groups of chemotherapeutic drugs: melphalan and doxorubicin. What is the mechanism of this effect? Recently, it was demonstrated in MM cells that the alkylating agent melphalan was able to dramatically down-regulate Mcl-1 and Bcl-xL and disrupt the Mcl-1/Bim complex.47 It was proposed that in MM cells Noxa may also displace Bim from Mcl-1.48 Thus, GSI may synergize with melphalan in Bim release. Doxorubicin and mitoxantrone are DNA intercalating agents and are known to stabilize topoisomerase II–DNA complexes.49 Drug-induced DNA–topoisomerase II complexes are referred to as “cleavable complexes,” and available evidence indicates that this is the initiating event in the cytotoxic effects of these drugs.50 It was shown that reducing Bim levels confers resistance to mitoxantrone in K562 cells.51 In MCF7 cells, apoptosis induced by doxorubicin was overcome by overexpression of Bcl-2, suggesting that this protein is at least one of the mediators of doxorubicin-induced cell death.52,53 Doxorubicin activates caspase 3, 8, and 9 in both oral squamous cell carcinoma HSC-2 and HL-60 cell lines.54 Thus, the ability of GSI to up-regulate Noxa and displace Bak and Bax may eliminate potential mechanisms that reduce the effect of cytotoxic drugs.
The data presented here demonstrate that the inhibition of Notch signaling in MM cells may not only induce apoptosis of MM cells but may also substantially enhance the effect of chemotherapy. This warrants further investigation of the potential clinical utility of this approach for the treatment of MM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kim Paraiso, Daniela Wood, and William Kerr for assistance in performing experiments with SCID-hu mice and Anthony M. Neuger in evaluation of GSI serum concentration.
This work was supported in part by a Multiple Myeloma Research Foundation Senior Research Award and Bankhead Coley Grant from the State of Florida (D.I.G.).
Authorship
Contribution: Y.N. designed and performed experiments and wrote the paper; D.M.S. provided patient samples and helpful suggestions; S.C.B. performed experiments; W.S.D. discussed the results and provided helpful suggestions; and D.I.G. designed the experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yulia Nefedova, H. Lee Moffitt Cancer Center, 12902 Magnolia Dr, MRC 2067, Tampa, FL 33612; e-mail: julia.nefedova@moffitt.org.