Abstract
Diffuse large B-cell lymphoma (DLBCL) is an aggressive and the most common type of non-Hodgkin lymphoma. Despite recent advances in treatment, less than 50% of the patients are cured with current multiagent chemotherapy. Abnormal NF-κB activity not only contributes to tumor development but also renders cancer cells resistant to chemotherapeutic agents. Identifying and targeting signaling molecules that control NF-κB activation in cancer cells may thus yield more effective therapy for DLBCL. Here, we show that while overexpression of protein kinase C–associated kinase (PKK) activates NF-κB signaling in DLBCL cells, suppression of PKK expression inhibits NF-κB activity in these cells. In addition, we show that NF-κB activation induced by B cell–activating factor of tumor necrosis factor family (BAFF) in DLBCL cells requires PKK. Importantly, we show that knockdown of PKK impairs the survival of DLBCL cells in vitro and inhibits tumor growth of xenografted DLBCL cells in mice. Suppression of PKK expression also sensitizes DLBCL cells to treatment with chemotherapeutic agents. Together, these results indicate that PKK plays a pivotal role in the survival of human DLBCL cells and represents a potential target for DLBCL therapy.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is an aggressive and the most common subtype of non-Hodgkin lymphoma, accounting for 30% to 40% of lymphoid malignancy. Although DLBCL represents one of the most therapy-responsive malignancies, only approximately 40% of the patients can be cured by the current treatments with multiple therapeutic agents.1,2 As a result, nearly 10 000 patients die of DLBCL each year in the United States,2 underscoring the importance of better molecular understanding of DLBCL and identifying proper therapeutic targets. On the basis of gene expression profiles, DLBCL can be divided into at least 3 subgroups: activated B-cell–like (ABC) DLBCL, germinal center B cell–like (GCB) DLBCL, and primary mediastinal B-cell lymphoma (PMBL), with the ABC and PMBL subgroups exhibiting higher levels of expression of NF-κB target genes than the GCB subgroup.3-6 It was shown that constitutive NF-κB signaling is required for survival of ABC and PMBL DLBCL cells7,8 and that small molecules that inhibit IκB kinases (IKKs) are selectively toxic for these 2 DLBCL subgroup cells.9 These studies highlight the NF-κB pathway as a promising therapeutic target in B-cell lymphomas that depend on NF-κB activity for proliferation and survival.
The NF-κB family consists of 5 members in mammals: p65 (RelA), RelB, c-Rel, NF-κB1(p105/p50), and NF-κB2(p100/p52). Members of NF-κB proteins form homodimers or heterodimers and are retained in the cytoplasm before activation by the associated IκB proteins or the precursor proteins p100 or p105, which contain the ankyrin repeats present also in the IκB proteins and thus can also function as IκB proteins. Extracellular signals induce NF-κB activation through 2 major pathways: the classical (also called the canonical pathway) and the alternative (the noncanonical) pathways. Activation of these pathways leads to activation of IKK and degradation or processing of IκB proteins, resulting in release of sequestered NF-κB proteins, their subsequent translocation into the nucleus and target gene activation. While activation of the canonical NF-κB pathway predominantly results in release of active p50/p65 and p50/C-Rel dimers, the alternative pathway leads to nuclear accumulation of the p52/RelB complex.10-12 Abnormal activation of NF-κB contributes to tumor development and progression, as well as to the resistance of cancer cells to chemotherapeutic agents and radiation therapy.13,14 Thus, inhibition of NF-κB activation represents a promising approach for cancer therapy.13-15 However, as NF-κB is expressed ubiquitously and is involved in a wide variety of normal cellular functions, a nonselective inhibition of NF-κB activity would likely cause serious side effects.13,16,17 To achieve the specificity required for effective therapeutic intervention, it may be necessary to target cancer cell- or signal-specific regulators of NF-κB activation pathways.
The B cell–activating factor belonging to the tumor necrosis factor family (BAFF, also known as BlyS, TALL-1, THANK, zTNF-4, CD257, and TNFSF-13B) is critical for the development and survival of normal B lymphocytes and activates NF-κB through both the classical and the alternative activation pathways in B cells.18-22 Dysregulated BAFF signaling has been associated with various B-cell malignancies. Elevated levels of BAFF have been detected in the serum of patients with various types of B-cell malignancies, and malignant B cells from patients express abnormally high levels of BAFF and one or more BAFF receptors.23-29 It has been shown that BAFF functions as a crucial autocrine and paracrine survival factor for malignant B cells,21,23,25,27-31 making BAFF signaling pathway an attractive therapeutic target for B-cell malignancies. Despite the importance of BAFF signaling in both normal and malignant B cells, the mechanisms by which BAFF activates downstream events, including NF-κB activation, remain to be elucidated.
Protein kinase C–associated kinase (PKK; also known as DIK and RIP4) was initially identified as a protein kinase C β– and δ–interacting protein.32,33 It belongs to the RIP kinase family and shares high sequence homology at the N-terminal kinase domain with other members of this kinase family but contains unique C-terminal ankyrin repeats.34 Mice deficient in PKK die soon after birth, probably because of suffocation caused by abnormal epidermal differentiation.35 We and others previously showed that PKK activates NF-κB when overexpressed in nonlymphoid cells.36-38 However, the role of PKK in NF-κB activation induced by signaling receptors, as well as the function of PKK in the proliferation or survival of cancer cells, is unknown.
In this study, we investigated the requirement for PKK in NF-κB activation and survival in human DLBCL cells. We found that PKK regulates NF-κB activation in DLBCL cells and identified BAFF as one of the upstream signaling molecules that requires PKK for NF-κB activation in these cells. Inhibition of PKK expression impairs survival of DLBCL cells in vitro and tumor growth of implanted DLBCL cells in immune-deficient mice, as well as sensitizes DLBCL cells to treatment with chemotherapeutic agents.
Methods
Cell culture, antibodies, and reagents
Four DLBCL cell lines, OCI-Ly10, OCI-Ly3 (ABC-like DLBCL), OCI-Ly7, and SUDHL-6 cells (GC-like DLBCL), were cultured as described,8 except that fetal bovine serum (10% or as indicated) was used. To generate SUDHL-6 cells that stably express the N-terminal kinase domain (PKK-N, amino acids 1-320) of PKK,33,36 the cells were infected with pMIG retroviral vector (generously provided by Dr Warren Pear, University of Pennsylvania) that expresses Flag-tagged PKK-N and GFP. PKK-N–expressing cells were then sorted through GFP expression. Human BAFF was purchased from PeproTech (Rocky Hill, NJ). Antibodies against human IKKα, IKKβ, p65, p50, c-Rel, RelB, and PARP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–human p52 antibody was from Upstate Biotechnology (Charlottesville, VA). Antibodies specific for IκBα, phospho-IκBα (p-Ser32), phospho-IKKα (p-Ser176/180)/IKKβ (p-Ser177/181), and cleaved casepase3 were from Cell Signaling Technology (Danvers, MA). Antibody specific for Flag tag (M2) was from Sigma Chemical (St Louis, MO).
RNA interference using small hairpin RNA
PKK expression was suppressed by RNA interference with small hairpin RNAs (shRNAs) specific for human PKK mRNA. Three targeting sequences (shPKK-1: 5′-GGCCCACCTTCCAAGAAATTA; shPKK-2: 5′-CGTTCGTTTCTCGTTGCCTAA; and shPKK-3: 5′-GCACGATGTATACAGCTTTGC) were selected and cloned into either the retroviral pRetro-H1G vector (referred to as shPKK-1, shPKK-2, and shPKK-3, respectively) or the lentiviral vector plenty-hu6BX (referred to as L-shPKK-1, L-shPKK-3, and L-shPKK-3, respectively [Cellogenetics, Ijamsville, MD]). A random hairpin sequence (5′-GTTCTCCGAACGTGTCACG) was also cloned into these 2 vectors and referred to as shControl and L-shControl, respectively. Lentiviruses expressing L-shControl or an L-shPKK were produced using ViraPower lentiviral expression system following the protocol suggested by the manufacturer (Invitrogen, Carlsbad, CA). To generate SUDHL-6 or OCI-Ly7 cells that stably express shControl or an shPKK, the cells were infected with the recombinant retroviruses, produced using amphotropic viral packaging cell line (kindly provided by Dr Gary Nolan, Stanford University). The transduced cells were selected with puromycin (0.5 μg/mL) for 2 weeks. After the drug selection, bulk infected cells were used in the experiments to avoid clonal variations.
To assess the efficiency of PKK knockdown by the PKK-specific shRNA constructs, total RNA was isolated using RNeasy kit (QIAGEN, Valencia, CA), and the standard reverse transcriptase–polymerase chain reaction (RT-PCR) procedure was performed using the reagents from Invitrogen. The primers (5′-ATGGAGGGCGACGGCGGGACC-3′, and 5′-CAGGGAGCCCGTCTCCATGT-3′) specific for human PKK were used for PCR reaction.
Transfection, immunoprecipitation, Western blot analysis, and in vitro kinase assays
Preparation of cell lysates, immunoprecipitation, and Western blot analysis were performed as previously described.33,39 For in vitro kinase assays, the lysates from SUDHL-6 expressing shControl and shPKK-1, respectively, were immunoprecipitated with an antibody specific for human IKKβ. Half of the immunoprecipitates were used for analyzing the amounts of immunoprecipitated IKKβ by Western blotting. The other half was used for in vitro kinase assays, which were performed in the presence of [γ-32P]ATP essentially as previously described.33,39 Purified GST-IκBα (amino acids 1-54) was used as the substrate for the precipitated IKK complex. The kinase reaction products were analyzed by autoradiography after SDS–polyacrylamide gel electrophoresis.
Analysis of NF-κB DNA-binding activity
The DNA-binding activity of NF-κB subunits was analyzed using an enzyme-linked immunoabsorbent assay (ELISA)–based assay according to the manufacturer's instructions (TransAM NF-κB family Transcription Factor assay kit; Active Motif, Carlsbad, CA). Briefly, nuclear extracts were added into the wells of a 96-well plate that contain the immobilized oligonucleotide carrying an NF-κB consensus site. NF-κB proteins bound to this immobilized oligonucleotide were detected by incubating with a primary antibody that recognizes active p50, p52, p65, C-Rel, or RelB, followed by HRP-conjugated secondary antibody, and quantified by spectrophotometry at 450 nm with a reference wavelength of 650 nm. Statistic significance of the difference in NF-κB DNA-binding activity from different samples was analyzed by standard Student t test.
Cell viability and apoptosis assays
Cell viability was measured by the MTT assays as described by the manufacturer (Roche, Indianapolis, IN). The apoptosis assays were performed using the annexin V-PE apoptosis detection kit I (BD Bioscience, San Jose, CA). At least 10 000 events were recorded. The fluorescence-activated cell sorting (FACS) data were analyzed using Flojo5.1 software (BD Biosciences, San Jose, CA).
In vivo xenografts
All animal experiments were performed in compliance with institutional guidelines and with approval of our Institutional Animal Care and Use Committee. Nonobese diabetic/severe combined immunodeficient (NOD/SCID) male mice age 6 to 8 weeks were obtained from The Jackson Laboratory (Bar Harbor, ME). OCI-Ly7 cells (5 × 106) stably expressing shControl or shPKK-1, respectively, were injected subcutaneously into NOD/SCID mice (n = 4, each). The tumor size was measured at different days after implantation. The volumes of the tumors were calculated according to the formula: tumor volume = 4/3π × (length/2) × (width/2) × (height). Statistic significance of the difference was analyzed by standard Student t test.
Results
PKK regulates NF-κB activity in human DLBCL cells
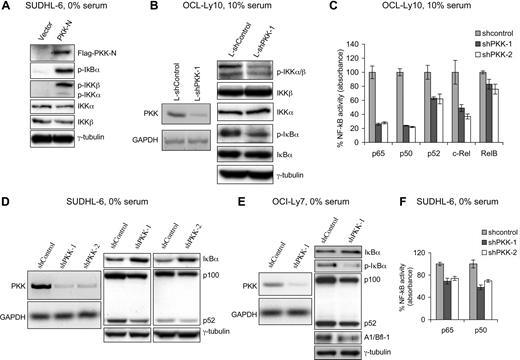
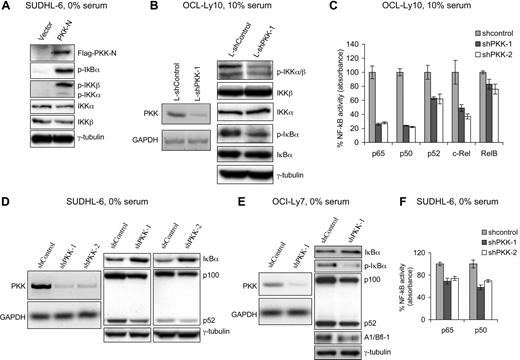
To investigate whether PKK regulates NF-κB activity in DLBCL cells, we first examined the effect of PKK overexpression on NF-κB activation in SUDHL-6 cells, a human GCB DLBCL cell line that has previously been shown to have low constitutive NF-κB activity.8 SUDHL-6 cells that stably express N-terminal kinase domain of PKK (PKK-N), which was previously shown to be capable of activating NF-κB reporter,36 exhibit higher levels of IκBα phosphorylation (Figure 1A), a hallmark of NF-κB activation.10-12 In addition, PKK-expressing cells showed higher levels of IKK phosphorylation at the 2 conserved serine residues (Ser176 and Ser180 in IKKα and Ser177 and Ser181 in IKKβ) (Figure 1A), which is known to be required for IKK activation.40-42 These results indicate that PKK can activate NF-κB in DLBCL cells.
PKK regulates NF-κB activity in DLBCL cells. (A) Overexpression of PKK activates NF-κB in DLBCL cells. SUDHL-6 cells stably carrying pMIG vector or expressing Flag-tagged PKK-N were cultured in serum-free medium for 20 hours. The expression of the indicated proteins involved in NF-κB signaling was analyzed by Western blotting. p-IκBα, p-IKKα, and p-IKKβ represent phosphorylated forms of IκBα, IKKα, and IKKβ, respectively. Analysis of γ-tubulin was used as the equal loading control. (B) Suppression of PKK expression inhibits NF-κB activity in ABC DLBCL cells. OCI-Ly10 cells were infected with lentiviruses expressing either a control shRNA (L-shControl) or a PKK-specific shRNA (L-shPKK-1). Three days after infection, RNA levels of PKK in the indicated cells were analyzed by RT-PCR (left), and expression of the indicated proteins involved in NF-κB signaling was analyzed by Western blotting (right). Analysis of GAPDH and γ-tubulin was used as loading controls. (C) PKK knockdown leads to inhibition of NF-κB DNA-binding activity in ABC DLBCL cells. OCI-Ly10 cells were infected with lentiviruses expressing a control shRNA (L-shControl) or a PKK-specific shRNA (shPKK-1 or shPKK-2). Sixty-four hours after infection, nuclear extracts were prepared and the DNA-binding activity of the indicated NF-κB subunit was analyzed as described in “Methods.” The assays were performed in triplicates. shControl-expressing cells contain significantly higher NF-κB DNA-binding activity than cells expressing an shPKK (P < 0.01 for all NF-κB subunits, except for RelB subunit). (D) Suppression of PKK expression results in decreased NF-κB activity in GCB DLBCL cell line SUDHL-6 cells. SUDHL-6 cells stably expressing either the control shRNA (shControl) or a PKK-specific shRNA (shPKK-1 or shPKK-2) were cultured in serum-free medium overnight. The levels of PKK mRNA in the indicated cells were analyzed by RT-PCR (right), and the expression of the indicated proteins was analyzed on Western blots (right). (E) Suppression of PKK expression inhibits NF-κB activity in GCB DLBCL cell line OCI-Ly7 cells. OCI-Ly7 cells stably expressing either shControl or shPKK-1 were cultured in serum-free medium overnight. Expression of PKK mRNA (left) and the indicated proteins involved in NF-κB signaling (right) were analyzed as described in panel D. (F) PKK knockdown reduces NF-κB DNA-binding activity in GCB DLBCL cells. SUDHL-6 cells stably expressing the indicated shRNA were cultured in serum-free medium overnight. Nuclear extracts were prepared, and the DNA-binding activity of p65 and p50 were assayed as described in panel C. The difference between control cells and cells expressing an shPKK in NF-κB DNA-binding activity is statistically significant (P < .01).
PKK regulates NF-κB activity in DLBCL cells. (A) Overexpression of PKK activates NF-κB in DLBCL cells. SUDHL-6 cells stably carrying pMIG vector or expressing Flag-tagged PKK-N were cultured in serum-free medium for 20 hours. The expression of the indicated proteins involved in NF-κB signaling was analyzed by Western blotting. p-IκBα, p-IKKα, and p-IKKβ represent phosphorylated forms of IκBα, IKKα, and IKKβ, respectively. Analysis of γ-tubulin was used as the equal loading control. (B) Suppression of PKK expression inhibits NF-κB activity in ABC DLBCL cells. OCI-Ly10 cells were infected with lentiviruses expressing either a control shRNA (L-shControl) or a PKK-specific shRNA (L-shPKK-1). Three days after infection, RNA levels of PKK in the indicated cells were analyzed by RT-PCR (left), and expression of the indicated proteins involved in NF-κB signaling was analyzed by Western blotting (right). Analysis of GAPDH and γ-tubulin was used as loading controls. (C) PKK knockdown leads to inhibition of NF-κB DNA-binding activity in ABC DLBCL cells. OCI-Ly10 cells were infected with lentiviruses expressing a control shRNA (L-shControl) or a PKK-specific shRNA (shPKK-1 or shPKK-2). Sixty-four hours after infection, nuclear extracts were prepared and the DNA-binding activity of the indicated NF-κB subunit was analyzed as described in “Methods.” The assays were performed in triplicates. shControl-expressing cells contain significantly higher NF-κB DNA-binding activity than cells expressing an shPKK (P < 0.01 for all NF-κB subunits, except for RelB subunit). (D) Suppression of PKK expression results in decreased NF-κB activity in GCB DLBCL cell line SUDHL-6 cells. SUDHL-6 cells stably expressing either the control shRNA (shControl) or a PKK-specific shRNA (shPKK-1 or shPKK-2) were cultured in serum-free medium overnight. The levels of PKK mRNA in the indicated cells were analyzed by RT-PCR (right), and the expression of the indicated proteins was analyzed on Western blots (right). (E) Suppression of PKK expression inhibits NF-κB activity in GCB DLBCL cell line OCI-Ly7 cells. OCI-Ly7 cells stably expressing either shControl or shPKK-1 were cultured in serum-free medium overnight. Expression of PKK mRNA (left) and the indicated proteins involved in NF-κB signaling (right) were analyzed as described in panel D. (F) PKK knockdown reduces NF-κB DNA-binding activity in GCB DLBCL cells. SUDHL-6 cells stably expressing the indicated shRNA were cultured in serum-free medium overnight. Nuclear extracts were prepared, and the DNA-binding activity of p65 and p50 were assayed as described in panel C. The difference between control cells and cells expressing an shPKK in NF-κB DNA-binding activity is statistically significant (P < .01).
We next determined whether PKK is required for NF-κB activation in DLBCL cells. For this purpose, we examined the effect of suppression of PKK on NF-κB activity in OCI-Ly10 cells, a human ABC DLBCL cell line that had been shown to exhibit high constitutive NF-κB activity.8 We prepared a recombinant lentivirus that expresses a PKK-specific shRNA (L-shPKK-1) and the green fluorescent protein (GFP). As a control, we also prepared a lentivirus that expresses a random hairpin sequence and the GFP protein (L-shControl). Forty-eight hours after lentiviral infection, approximately 70% to 80% of the OCI-Ly10 cells were GFP positive, and the 2 viruses yield comparable infection efficiency (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The expression of PKK was effectively suppressed in the OCI-Ly10 cells by the L-shPKK-1 (Figure 1B). Suppression of PKK expression resulted in inhibition of NF-κB activity in OCI-Ly10 cells as indicated by the decreases in IκBα phosphorylation as well as IKK phosphorylation (Figure 1B). To gain more direct evidence that PKK is required for NF-κB activity in OCI-Ly10 cells, we compared NF-κB DNA-binding activity in control cells and the cells expressing a PKK-specific shRNA. In these experiments, we included an additional shPKK, L-shPKK-2, that targets a different PKK mRNA sequence from the one targeted by L-shPKK-1 and effectively inhibited PKK expression (Figure S2A), so that the possibility of the “off-target” effect from the use of a single shPKK could be minimized. PKK knockdown by either shPKK led to significant decreases in the DNA-binding activity of several members of the NF-κB family (Figure 1C). The decreases in DNA-binding activity correlated with the reduced nuclear levels of the NF-κB proteins in shPKK-expressing cells (Figure S2B). These results thus show that PKK is required for NF-κB activation in ABC DLBCL cells. Consistent with the observation that PKK overexpression induced IKK phosphorylation, PKK knockdown inhibited IKK phosphorylation (Figure 1B), indicating that PKK regulates NF-κB activation through modulating IKK activation.
We also investigated whether PKK is required for NF-κB activity in GCB DLBCL cells. To knockdown the expression of PKK in SUDHL-6 and OCI-Ly7 cells, 2 GCB DLBCL cell lines, we generated the cells that stably express either a small hairpin RNA specific for PKK (shPKK-1 and shPKK-2) or a control small hairpin RNA (shControl). Expression of an shPKK effectively inhibited expression of PKK in both SUDHL-6 and OCI-Ly7 cells (Figure 1D,E). Suppression of PKK expression apparently had little effect on the constitutive NF-κB activity in these cells cultured in the normal growth (10% serum) medium (data not shown). In contrast, PKK knockdown in these cells cultured in serum-free medium yielded a moderate, but reproducible, decrease in the basal NF-κB activity, as suggested by the increase in IκBα protein level and the decreases in the expression of several known NF-κB targets43-46 (Figure 1D,E; Figure S3). Consistent with the decreased expression of NF-κB targets, the DNA binding activity of p65 and p50 in the serum-starved cells is inhibited after PKK knockdown (Figure 1F). Thus, PKK is also required for basal NF-κB activity in GCB DLBCL cells. The lack of apparent effect of PKK knockdown on NF-κB activity in SUDHL-6 and OCI-Ly7 cells cultured in 10% serum may reflect that PKK regulates NF-κB activation by some but not all signaling pathways in these cells. Taken together, the above results indicate that PKK regulates NF-κB activation in DLBCL cells and that PKK functions upstream of IKK activation.
PKK regulates NF-κB activation induced by BAFF in DLBCL cells
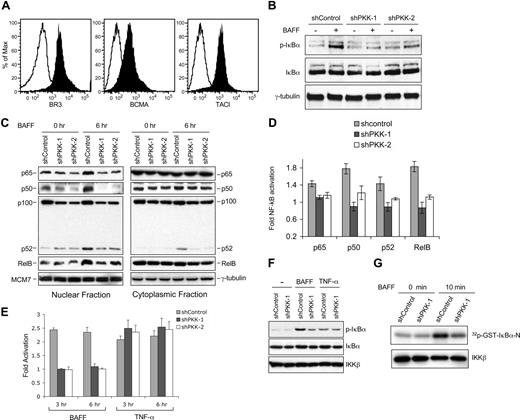
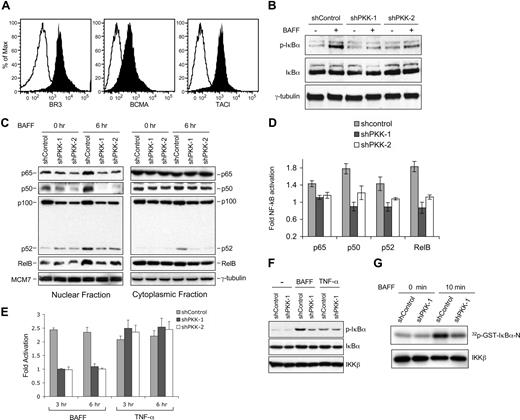
Having shown that PKK regulates NF-κB activation in DLBCL cells, we then asked which stimulus requires PKK for NF-κB activation in DLBCL cells. The observation that PKK knockdown inhibited NF-κB activity in cells cultured in serum-free media suggests that PKK may regulate NF-κB activation in these cells by an autocrine signal(s). Because BAFF induces NF-κB activation in both normal and malignant B lymphocytes, and a variety of malignant B cells produce BAFF as a critical autocrine survival factor,21,23,25,27,30 we explored whether PKK regulates NF-κB activation induced by BAFF in DLBCL cells. SUDHL-6 cells express all 3 known receptors for BAFF47 (Figure 2A). BAFF treatment of SUDHL-6 cells expressing the control shRNA (shControl) led to increases in IκBα phosphorylation (Figure 2B), nuclear translocation of NF-κB proteins (Figure 2C), NF-κB DNA-binding activity (Figure 2D), and NF-κB–responsive reporter activation (Figure 2E), indicating the activation of NF-κB signaling. In contrast, such increases were severely inhibited in SUDHL-6 cells that express a PKK-specific shRNA (Figure 2B-E; Figure S4). Suppression of PKK expression in OCI-Ly7 similarly resulted in inhibition of BAFF-induced NF-κB activation (Figure S4), suggesting that requirement for PKK in BAFF-induced NF-κB activation is not limited to SUDHL-6 cells.
PKK regulates NF-κB activation induced by BAFF. (A) Surface expression of the indicated BAFF receptors in SUDHL-6 cells. SUDHL-6 cells were incubated with antibodies specific for human BAFF receptor (BR3 or BAFF-R), B cell–maturation antigen (BCMA), or transmembrane activator and calcium modulator cyclophilin ligand interactor (TACI) (filled histograms) or a control antibody (open histograms). After incubated with secondary antibodies, the expression of the indicated BAFF receptors was analyzed by flow cytometry.30 (B) Suppression of PKK expression inhibits I-κBα phosphorylation induced by BAFF. SUDHL-6 cells stably expressing shControl, shPKK-1, or shPKK-2 were treated with BAFF (200 ng/mL) for 15 minutes in serum-free medium. The expression levels of the indicated proteins were analyzed by Western blotting. The shRNA-expressing SUDHL-6 cells used here are the same cells described in Figure 1D. The PKK knockdown efficacy was confirmed to be at the levels shown in Figure 1D. (C) PKK knockdown leads to inhibition of nuclear translocation of NF-κB proteins. SUDHL-6 cells stably expressing the indicated shRNA were treated with BAFF (500 ng/mL) for 6 hours in serum-free medium. Nuclear and cytoplasmic fractions were prepared according to the instructions by the manufacturer (Active Motif), and the levels of the indicated NF-κB subunits in each fraction were analyzed by Western blotting. Analysis of MCM7 and γ-tubulin was used as the loading controls for nuclear and cytoplasmic fractions, respectively. (D) Suppression of PKK expression inhibits BAFF-induced NF-κB DNA-binding activity. SUDHL-6 cells expressing the indicated shRNA were treated as described in panel C, and the DNA-binding activity of the indicted NF-κB subunit was assayed as described in Figure 1C. The difference between control cells and cells expressing an shPKK in DNA-binding activity for all tested NF-κB subunits is significant (P < .01). (E) Effect of PKK knockdown on NF-κB promoter activation induced by BAFF and TNF-α. SUDHL-6 cells stably expressing the indicated shRNA were infected with a recombinant lentivirus carrying a firefly luciferase reporter regulated by 4-tandam copies of NF-κB consensus binding sequence (generously provided by John Ashton, University of Rochester). Forty hours after infection, the cells were treated with BAFF (500 ng/mL) and TFN-α (100 ng/mL), respectively, for the indicated times. The luciferase activity was measured as described previously.36 Fold of activation was calculated by comparing the luciferase activity of BAFF or TNF-α stimulated cells with that of unstimulated cells. The means and standard deviations from 3 independent experiments are depicted. (F) Suppression of PKK expression inhibits BAFF-, but not TNF-α–, induced IκBα phosphorylation. SUDHL-6 cells stably expressing the indicated shRNA were treated with BAFF and TNF-α, respectively, for 3 minutes. The expression levels of the indicated proteins were analyzed by Western blotting. Analysis of IKKβ was used as a loading control. (G) SUDHL-6 cells expressing either shControl or shPKK-1 were treated with BAFF for the indicated time. IKK complex was immunoprecipitated with an IKKβ-specific antibody. The kinase activity of the immunoprecipitated IKK complex was assayed in vitro using GST-IκBα (amino acids 1-54) as a substrate (top). The amounts of IKKβ protein in the immunoprecipitated samples were analyzed by Western blotting using an IKKβ antibody (bottom).
PKK regulates NF-κB activation induced by BAFF. (A) Surface expression of the indicated BAFF receptors in SUDHL-6 cells. SUDHL-6 cells were incubated with antibodies specific for human BAFF receptor (BR3 or BAFF-R), B cell–maturation antigen (BCMA), or transmembrane activator and calcium modulator cyclophilin ligand interactor (TACI) (filled histograms) or a control antibody (open histograms). After incubated with secondary antibodies, the expression of the indicated BAFF receptors was analyzed by flow cytometry.30 (B) Suppression of PKK expression inhibits I-κBα phosphorylation induced by BAFF. SUDHL-6 cells stably expressing shControl, shPKK-1, or shPKK-2 were treated with BAFF (200 ng/mL) for 15 minutes in serum-free medium. The expression levels of the indicated proteins were analyzed by Western blotting. The shRNA-expressing SUDHL-6 cells used here are the same cells described in Figure 1D. The PKK knockdown efficacy was confirmed to be at the levels shown in Figure 1D. (C) PKK knockdown leads to inhibition of nuclear translocation of NF-κB proteins. SUDHL-6 cells stably expressing the indicated shRNA were treated with BAFF (500 ng/mL) for 6 hours in serum-free medium. Nuclear and cytoplasmic fractions were prepared according to the instructions by the manufacturer (Active Motif), and the levels of the indicated NF-κB subunits in each fraction were analyzed by Western blotting. Analysis of MCM7 and γ-tubulin was used as the loading controls for nuclear and cytoplasmic fractions, respectively. (D) Suppression of PKK expression inhibits BAFF-induced NF-κB DNA-binding activity. SUDHL-6 cells expressing the indicated shRNA were treated as described in panel C, and the DNA-binding activity of the indicted NF-κB subunit was assayed as described in Figure 1C. The difference between control cells and cells expressing an shPKK in DNA-binding activity for all tested NF-κB subunits is significant (P < .01). (E) Effect of PKK knockdown on NF-κB promoter activation induced by BAFF and TNF-α. SUDHL-6 cells stably expressing the indicated shRNA were infected with a recombinant lentivirus carrying a firefly luciferase reporter regulated by 4-tandam copies of NF-κB consensus binding sequence (generously provided by John Ashton, University of Rochester). Forty hours after infection, the cells were treated with BAFF (500 ng/mL) and TFN-α (100 ng/mL), respectively, for the indicated times. The luciferase activity was measured as described previously.36 Fold of activation was calculated by comparing the luciferase activity of BAFF or TNF-α stimulated cells with that of unstimulated cells. The means and standard deviations from 3 independent experiments are depicted. (F) Suppression of PKK expression inhibits BAFF-, but not TNF-α–, induced IκBα phosphorylation. SUDHL-6 cells stably expressing the indicated shRNA were treated with BAFF and TNF-α, respectively, for 3 minutes. The expression levels of the indicated proteins were analyzed by Western blotting. Analysis of IKKβ was used as a loading control. (G) SUDHL-6 cells expressing either shControl or shPKK-1 were treated with BAFF for the indicated time. IKK complex was immunoprecipitated with an IKKβ-specific antibody. The kinase activity of the immunoprecipitated IKK complex was assayed in vitro using GST-IκBα (amino acids 1-54) as a substrate (top). The amounts of IKKβ protein in the immunoprecipitated samples were analyzed by Western blotting using an IKKβ antibody (bottom).
As a control, we also examined the requirement for PKK in NF-κB singling activated by TNF-α. Interestingly, although NF-κB activation induced by BAFF requires PKK, NF-κB activation induced by TNF-α appears to be PKK independent (Figure 2E-F). This observation supports our earlier proposal that PKK regulates NF-κB activation by some but not all signaling pathways in DLBCL cells. Consistent with the suggestion that PKK functions upstream of IKK activation, suppression of PKK expression inhibited IKK activation induced by BAFF in SUDHL-6 cells (Figure 2G). Together, these results show that PKK plays a critical role in NF-κB activation induced by BAFF in DLBCL cells.
PKK is essential for survival of ABC DLBCL cells
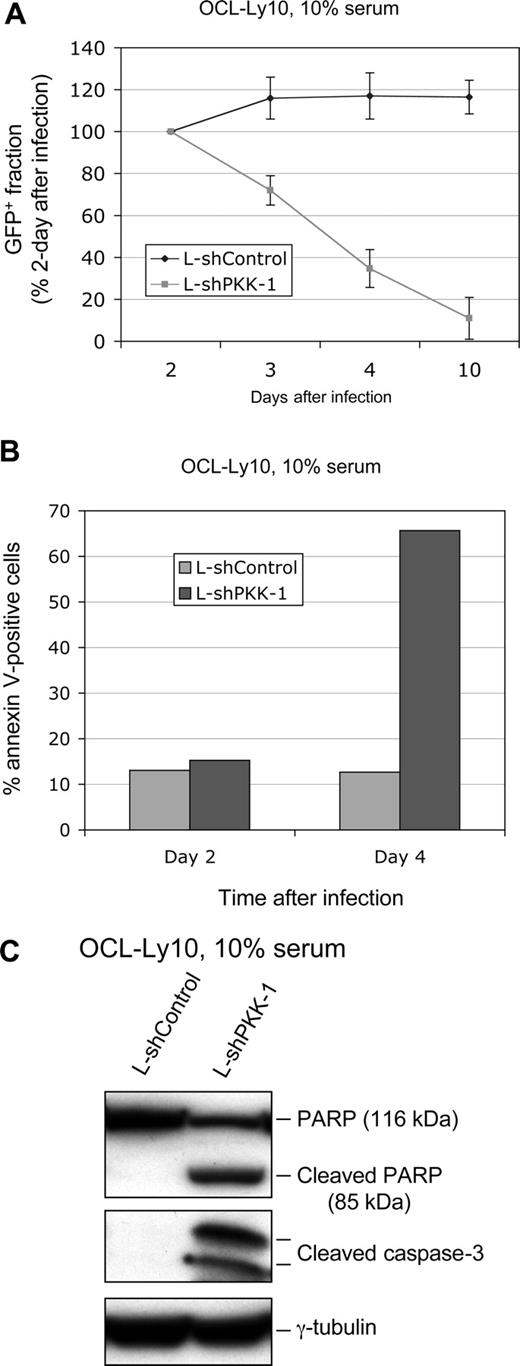
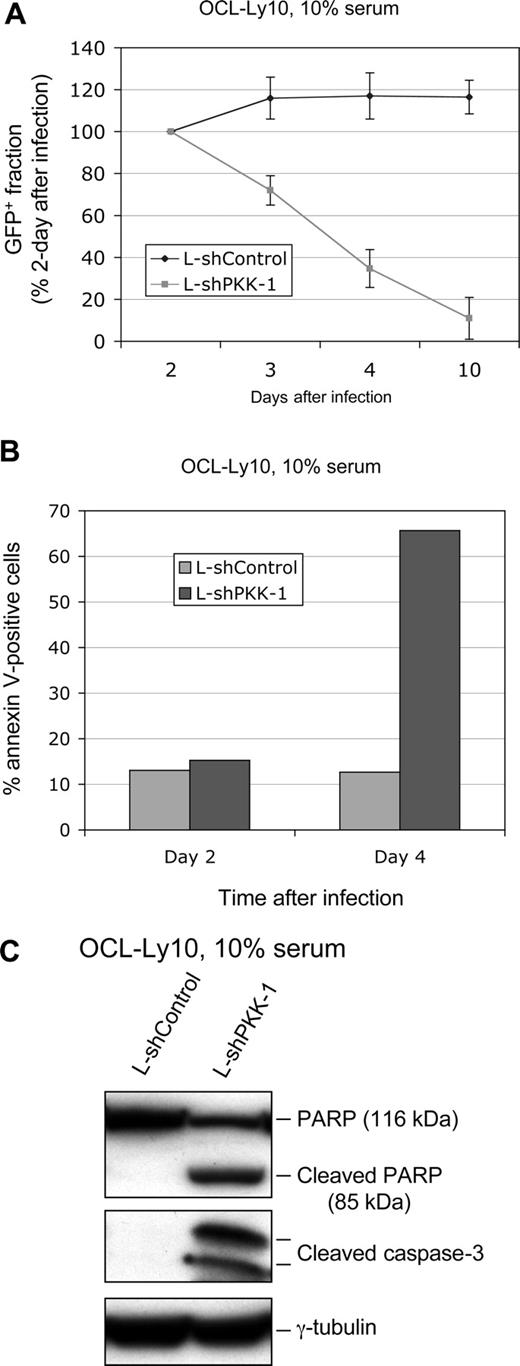
It has been shown that constitutive NF-κB activity is essential for survival of ABC DLBCL cells such as OCI-Ly10 cells.8,9 Given that PKK is crucial for NF-κB activity in these cells (Figure 1B), suppression of PKK expression would likely affect survival of these cells. To test this notion directly, we infected OCI-Ly10 cells with lentiviruses that express either L-shPKK-1 or L-shControl and examined survival of the infected cells. Because both viruses also express GFP, we monitored survival of the infected cells through flow cytometry analysis of fractions of GFP-positive cells over time after lentiviral infection. The fraction of GFP-positive cells that express L-shPKK-1 was significantly decreased by 3 days after infection, whereas no such decrease was observed for cells infected with the control virus (Figure 3A). The slight increase in the GFP-positive fraction in the control cells at 3 days after infection is probably due to GFP expression in additional cells after 2 days of viral infection. These results indicate that PKK is essential for survival of OCI-Ly10 cells. To confirm that loss of the GFP-positive cells that express L-shPKK resulted from cell death, we analyzed the fraction of annexin V–positive cells (apoptotic cells48,49 ) in the infected (GFP-positive) cells. As shown in Figure 3B, PKK knockdown significantly increased the fraction of annexin V–positive cells, further showing that PKK knockdown results in cell death of OCI-Ly10 cells. Consistent with the annexin V analysis, suppression of PKK expression in OCI-Ly10 cells by LshPKK-1 led to increases in PARP cleavage and activation of caspase-3 (cleaved caspase-3) (Figure 3C), hallmarks of apoptosis.50-52 PKK knockdown in OCI-Ly10 cells by another PKK-specific shRNA (L-shPKK-2) also led to cell death as indicated by the loss of L-shPKK-2–expressing cells (Figure S5A), suggesting that the cell death of OCI-Ly10 cells expressing a PKK-specific shRNA resulted from specific suppression of PKK expression rather than the nonspecific off-target effect of an shPKK. PKK knockdown also induced cell death of OCI-Ly3 cells, another ABC DLBCL cell line (Figure S5B). The reduced survival of ABC DLBCL cells infected with shPKK-expressing lentiviruses resulted most likely from specific inhibition of PKK expression, rather than from nonspecific toxicity generated by high-titer lentiviral transduction, because the lentivirus expressing the control shRNA yielded similar infection rate as the shPKK-expressing viruses and had no detectable effect on the survival of these cells (Figure 3; Figure S1). In addition, survival of the ABC DLBCL cells was significantly reduced by shPKK-expressing lentiviruses with wide-range (28%-85%) infection rates (Figures S1,S5; data not shown). Taken together, the results show that PKK is required for survival of ABC DLBCL cells by preventing the cells from apoptosis.
Suppression of PKK expression results in cell death of OCI-Ly10 cells. (A) OCI-Ly10 cells were infected with lentiviruses expressing either L-shControl or L-shPKK-1. At the indicated times after infection, the GFP-positive fractions were analyzed by flow cytometry. The GFP-positive fractions were normalized to the samples harvested at 2 days after infection. Means and SDs from 3 separate experiments are depicted. (B) OCI-Ly10 cells were infected as described in panel A, and the cell death was analyzed at 2 and 4 days after infection, respectively, using the annexinV-PE apoptosis detection kit (BD Bioscience). Shown are average results from 2 separate experiments. (C) OCI-Ly10 cells were infected as described in panel A. Three days after infection, expression of the indicated proteins was analyzed on Western blots.
Suppression of PKK expression results in cell death of OCI-Ly10 cells. (A) OCI-Ly10 cells were infected with lentiviruses expressing either L-shControl or L-shPKK-1. At the indicated times after infection, the GFP-positive fractions were analyzed by flow cytometry. The GFP-positive fractions were normalized to the samples harvested at 2 days after infection. Means and SDs from 3 separate experiments are depicted. (B) OCI-Ly10 cells were infected as described in panel A, and the cell death was analyzed at 2 and 4 days after infection, respectively, using the annexinV-PE apoptosis detection kit (BD Bioscience). Shown are average results from 2 separate experiments. (C) OCI-Ly10 cells were infected as described in panel A. Three days after infection, expression of the indicated proteins was analyzed on Western blots.
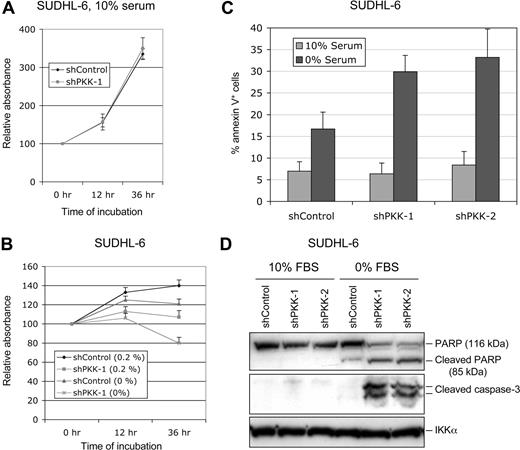
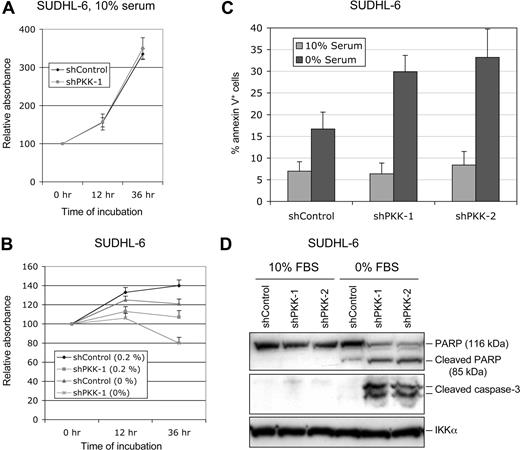
The observation that PKK is essential for survival of ABC DLBCL cells prompted us to investigate whether PKK is also involved in survival of GCB DLBCL cells. We cultured SUDHL-6 cells that stably express shControl or shPKK-1 in the medium containing different concentrations of serum. The survival of the cells was measured using MTT assay.53,54 Whereas PKK knockdown had little effect on the survival of SUDHL-6 cells cultured in medium containing 10% serum (Figure 4A; Figure S6), suppression of PKK expression impairs survival of these cells cultured in the medium with a low concentration of serum or without serum (Figure 4B). Similarly, PKK knockdown affected survival of OCI-Ly7 cells only when they were cultured with low concentrations of serum (Figures S6,S7). To gain direct evidence that suppression of PKK expression leads to cell death of GCB DLBCL cells when they were cultured at low concentrations of serum, we analyzed the cell death by the annexin V assay. As shown in Figure 4C, SUDHL-6 cells that express either the control shRNA (shControl) or a PKK-specific shRNA (shPKK-1 or shPKK-2) yielded similar fractions of annexin V–positive cells when cultured in the medium containing 10% serum. In contrast, the cells that express an shPKK exhibited much higher fractions of annexin V–positive cells than the control cells when they were cultured under the serum-free condition. In addition, PKK knockdown resulted in marked increases in the cleavage of PARP and activation of caspase-3 in SUDHL-6 cells when they were cultured without serum (Figure 4D). Thus, PKK is crucial for the survival of GCB DLBCL cells under certain conditions such as low serum conditions.
Effect of PKK knockdown on survival of SUDHL-6 cells. (A) SUDHL-6 cells stably expressing shControl or shPKK-1 were cultured in the medium containing 10% serum. The live cells were measured by the MTT assay. The results were normalized to the absorbance readings at the time the cells were plated (0 hour). Mean results and SDs from 3 experiments are shown. (B) The SUDHL-6 cells stably expressing shControl or shPKK-1 were cultured in the medium containing the indicated concentrations of serum. The relative live cells were analyzed as described in panel A at the indicated times. (C) SUDHL-6 cells stably expressing shControl or sh-PKK-1 were cultured in the medium containing 0% or 10% serum for 24 hours. The annexin V–positive cells (dying or dead) were analyzed as described in “Cell viability and apoptosis assays.” The data represent the mean results of 3 independent experiments. (D) The shRNA-expressing SUDHL-6 cells were cultured in the medium containing 0% or 10% serum for 24 hours. The cleavage of PARP and caspase 3 was analyzed on Western blots. The analysis of IKKα was used as the equal loading control.
Effect of PKK knockdown on survival of SUDHL-6 cells. (A) SUDHL-6 cells stably expressing shControl or shPKK-1 were cultured in the medium containing 10% serum. The live cells were measured by the MTT assay. The results were normalized to the absorbance readings at the time the cells were plated (0 hour). Mean results and SDs from 3 experiments are shown. (B) The SUDHL-6 cells stably expressing shControl or shPKK-1 were cultured in the medium containing the indicated concentrations of serum. The relative live cells were analyzed as described in panel A at the indicated times. (C) SUDHL-6 cells stably expressing shControl or sh-PKK-1 were cultured in the medium containing 0% or 10% serum for 24 hours. The annexin V–positive cells (dying or dead) were analyzed as described in “Cell viability and apoptosis assays.” The data represent the mean results of 3 independent experiments. (D) The shRNA-expressing SUDHL-6 cells were cultured in the medium containing 0% or 10% serum for 24 hours. The cleavage of PARP and caspase 3 was analyzed on Western blots. The analysis of IKKα was used as the equal loading control.
Requirement for PKK in tumor growth from xenografted GCB DLBCL cells
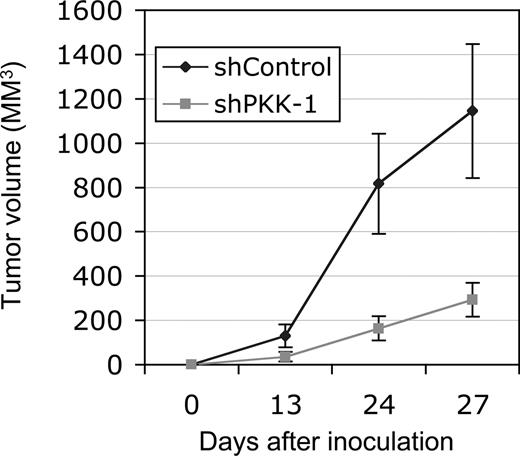
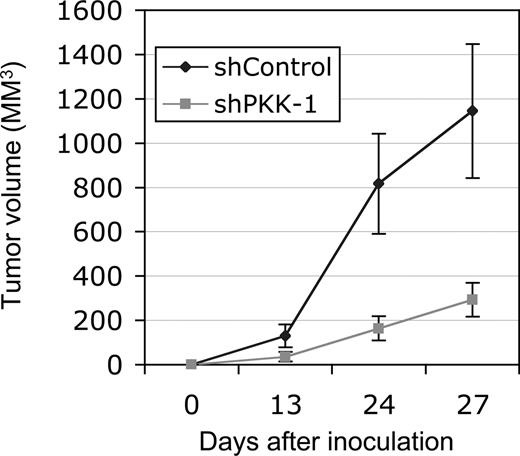
Because PKK knockdown affects the survival of DLBCL cells in vitro, we asked whether PKK is also required for the growth of these cells in vivo. We addressed this issue by examining the effect of PKK knockdown on tumor growth of DLBCL cells in immunodeficient mice. SUDHL-6 cells were unable to form tumors in NOD/SCID mice on subcutaneous inoculation under our experimental conditions (data not shown). We thus focused on tumor formation of xenografted OCI-Ly7 cells. Tumor growth was readily observed when OCI-Ly7 cells expressing the shControl were inoculated into the NOD/SCID mice. In contrast, the tumor growth of the OCI-Ly7 cells expressing shPKK-1 was significantly inhibited (Figure 5). Thus, PKK is also required for tumor growth of OCI-Ly7 cells in vivo at least in the described mouse model, possibly through modulating the survival of these cells in mice.
Suppression of PKK expression inhibits tumor growth of xenografted OCI-Ly7 cells in NOD/SCID mice. OCI-Ly7 cells expressing shControl or shPKK-1 were injected subcutaneously into NOD/SCID mice (n = 4, each). The tumor volumes were measured at the indicated times. The average tumor volumes from 4 injected mice were shown. The tumors from cells expressing shControl are significantly larger than those from the cells expressing shPKK-1 (P < .01 at day 27 after implantation).
Suppression of PKK expression inhibits tumor growth of xenografted OCI-Ly7 cells in NOD/SCID mice. OCI-Ly7 cells expressing shControl or shPKK-1 were injected subcutaneously into NOD/SCID mice (n = 4, each). The tumor volumes were measured at the indicated times. The average tumor volumes from 4 injected mice were shown. The tumors from cells expressing shControl are significantly larger than those from the cells expressing shPKK-1 (P < .01 at day 27 after implantation).
Knockdown of PKK expression sensitizes DLBCL cells to treatment with chemotherapeutic agents
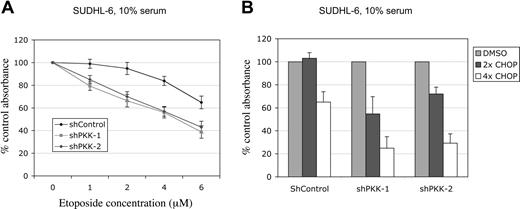
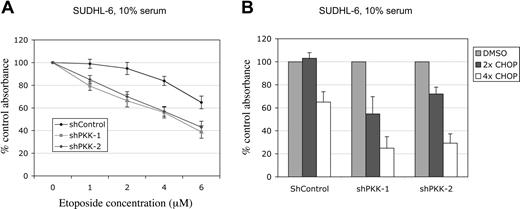
NF-κB activity protects cancer cells from apoptosis induced by chemotherapeutic agents and contributes to the resistance of malignant cells to chemotherapeutic agents.13,14 As our results showed that PKK plays a critical role in NF-κB activation in DLBCL cells, we reasoned that suppression of PKK expression might sensitize these cells to treatment by chemotherapeutic agents. To test this idea directly, we treated SUDHL-6 cells expressing the shControl or an shPKK with various concentrations of etoposide, a chemotherapeutic agent used in treatment of B-cell lymphomas.55,56 As shown in Figure 6A, PKK knockdown increased the sensitivity of SUDHL-6 cells to etoposide treatment. To determine whether the increased sensitivity by PKK knockdown is specific for etoposide or common to other chemotherapeutic agents, we also treated the cells with CHOP (a combination of cyclophosphamide, doxorubicin, vincristine, and prednisone), which has been widely used as the standard treatment for DLBCLs.1,57,58 Similar to the response to etoposide, SUDHL-6 cells expressing shPKK exhibited increased sensitivity to CHOP treatment (Figure 6B). Thus, inhibition of PKK expression sensitizes SUDHL-6 cells to treatment by multiple chemotherapeutic agents. These results suggest that PKK may contribute to the resistance of DLBCL cells to chemotherapeutic agents and inhibition of PKK may increase the efficacy of chemotherapy of DLBCL.
Suppression of PKK expression sensitizes DLBCL cells to treatment with chemotherapeutic agents. (A) SUDHL-6 cells expressing shControl or shPKK-1 were cultured in the medium containing 10% serum and treated with etoposide at the indicted concentrations for 36 hours. The relative live cells were analyzed by MTT assay. The absorbance readings from the cells treated with the vehicle (DMSO) were set at 100%. The mean results and SDs from 3 separate experiments are depicted. The difference between shPKK-expressing and the control cells was significant after the etoposide treatment at all concentrations tested (P < .01). (B) SUDHL-6 cells expressing shControl or an shPKK were treated with CHOP for 72 hours. The relative live cells were analyzed as described in panel A. The difference between the shPKK-expressing and the control cells was significant (P < .01, at each concentration). 1x CHOP consists of cyclophosphamide monophosphate, doxorubicin, vincristine, and prednisone at concentrations of 5.84pM, 1.5pM, 260pM, and 1.0μM, respectively.62
Suppression of PKK expression sensitizes DLBCL cells to treatment with chemotherapeutic agents. (A) SUDHL-6 cells expressing shControl or shPKK-1 were cultured in the medium containing 10% serum and treated with etoposide at the indicted concentrations for 36 hours. The relative live cells were analyzed by MTT assay. The absorbance readings from the cells treated with the vehicle (DMSO) were set at 100%. The mean results and SDs from 3 separate experiments are depicted. The difference between shPKK-expressing and the control cells was significant after the etoposide treatment at all concentrations tested (P < .01). (B) SUDHL-6 cells expressing shControl or an shPKK were treated with CHOP for 72 hours. The relative live cells were analyzed as described in panel A. The difference between the shPKK-expressing and the control cells was significant (P < .01, at each concentration). 1x CHOP consists of cyclophosphamide monophosphate, doxorubicin, vincristine, and prednisone at concentrations of 5.84pM, 1.5pM, 260pM, and 1.0μM, respectively.62
Discussion
Here, we show that PKK regulates constitutive NF-κB activity as well as NF-κB activation induced by BAFF in DLBCL cells. In addition, we show that PKK plays a crucial role in the survival of DLBCL cells in vitro and is required for the growth of these cells implanted in mice. We further show that suppression of PKK expression renders DLBCL cells more sensitive to treatment with chemotherapeutic agents. Thus, PKK is a critical regulator of NF-κB signaling in DLBCL cells and may represent a promising drug target for treatment of DLBCL and possibly other B-cell malignancies.
It was previously shown that survival of the ABC subgroup of DLBCL cells, but not the GCB subgroup, requires constitutive NF-κB activity when the cells were cultured under normal in vitro growth conditions.9,59 We have observed a good correlation between the effect of PKK knockdown on constitutive NF-κB activity in DLBCL cells (Figure 1; Figure S2) and its effect on DLBCL cell survival (Figures 3,4; Figure S5). These results are most consistent with the suggestion that PKK exerts its crucial role in the survival of DLBCL cells primarily through controlling NF-κB activation, although the possibility that PKK may control DLBCL cell survival through other signaling pathways has not been ruled out. Our finding that PKK is required for the survival of GCB DLBCL cells under lower serum conditions and is essential for the growth of these cells in mice (Figures 4B-D,5) extends previous observations and suggests that NF-κB activity also plays an important role in the survival of GCB DLBCL cells. Indeed, our preliminary results indicate that knockdown of IKKβ compromises the survival of GCB DLBCL cells under low-serum conditions (data not shown). Thus, GCB DLBCL subgroup may also be susceptible to the treatment with inhibitors of NF-κB signaling in vivo.
Although the results presented in this work clearly show that PKK is a crucial survival factor for DLBCL cells, the role of PKK in cell-cycle progression is less clear. Although PKK knockdown had little effect on the cell-cycle distribution of SUDHL-6 cells cultured in 10% serum, suppression of PKK expression modestly accelerated the decrease in S phase in serum-starved SUDHL-6 cells (Figure S8; data not shown), suggesting that PKK is also involved in the control of DLBCL cell proliferation. A detailed study on the role of PKK in cell-cycle progression, however, is hampered because PKK knockdown leads to rapid cell death. Regardless of the role of PKK in cell-cycle progression, it appears that PKK-mediated survival plays a dominant role in the maintenance of DLBCL cells.
PKK-deficient mice die within hours of birth, likely because of suffocation caused by abnormal epidermal differentiation.35 Analysis of B-cell compartment in PKK-null mice or in reconstituted Rag-1–null mice that lack PKK indicates that PKK is dispensable for normal mouse B-cell development.35,60 In addition, it was shown that NF-κB activation by BCR, CD40, and TLR-4 is not affected in PKK-deficient primary B cells.60 It is possible that PKK may also not play an essential role in BAFF signaling in normal mouse B cells, given that B-cell development is normal in PKK-null mice. Using shRNAs that target multiple distinct human PKK sequences, we show here that PKK plays a crucial role in constitutive NF-κB signaling, as well as in BAFF-induced NF-κB activation, and in the survival of DLBCL cells. The apparent difference between normal mouse B cells and human DLBCL cells in their requirement for PKK in NF-κB activation and survival suggest that PKK may have acquired an essential role(s) in DLBCL cells while it has only limited roles in the development of normal B cells in mice. Alternatively, the function of PKK in mouse B lymphocyte may be compensated by other members of RIP family whereas these members are unable to compensate the function of PKK in DLBCL cells. In this context, it may be worthy to note that PKK was inactivated in germ cells in the above-discussed mouse work, while PKK is acutely inactivated in somatic cells in our work. Future studies will be needed to distinguish these possibilities.
Emerging evidence indicates that BAFF plays a pivotal role in the survival of a variety of malignant B lymphocytes.21,61 Molecular understanding of BAFF signaling pathway may identify effective drug targets for the treatment of B-cell lymphomas. Here, we have shown that suppression of PKK inhibits NF-κB activation induced by BAFF in DLBCL cells (Figure 2; Figure S4). Thus, PKK may be an important player in BAFF signaling in malignant B cells. It was previously shown that BAFF induces NF-κB activation through both the canonical and the alternative pathways.18-22 Consistent with the previous studies, BAFF induced activation of both the canonical and the alternative NF-κB pathways in SUDHL-6 cells, as indicated by the observation that BAFF induced nuclear translocation and DNA-binding activity of p65 and p50 as well as those of p52 and RelB in these cells (Figure 2C,D). Our data clearly show that PKK is required for the activation of the canonical NF-κB pathway induced by BAFF in DLBCL cells (Figure 2; Figure S4). PKK knockdown in SUDHL-6 cells led to inhibition of BAFF-induced nuclear translocation and DNA-binding activity of p52 and RelB (Figure 2B-E; Figure S4), suggesting that PKK is apparently also involved in the activation of the alternative NF-κB pathway induced by BAFF in these cells. Because the expression of RelB and p100, the precursor of p52, is positively regulated by the canonical NF-κB pathway,43,44 and suppression of PKK inhibited expression of RelB and p100 (Figures 1D,E,2C; Figure S3), it is possible that the observed inhibition of the activation of RelB/p52 may result indirectly from the inhibition of the canonical pathway. Although the detailed mechanism underlying inhibition of RelB/p52 activation by PKK knockdown remains to be elucidated, it is clear that the overall NF-κB activity induced by BAFF in the DLBCL cells is severely compromised when PKK expression is suppressed (Figure 2; Figure S4).
BAFF binds to 3 known receptors BR3 (BAFF-R), BCMA, and TACI. While BR3 (BAFF-R) is required for BAFF-induced activation of the alternative NF-κB pathway, signaling through BMCA and TACI activates the canonical NF-κB pathway.21,30,47 SUDHL-6 cells express all 3 BAFF receptors, and BAFF activates both the canonical and the alternative NF-κB pathway in these cells (Figure 2). It is possible that all 3 BAFF receptors are involved in BAFF signaling in SUDHL-6 cells. At present, it is not clear which of the 3 BAFF receptors requires PKK for NF-κB activation. In this study, we showed that PKK is required for BAFF-induced NF-κB activation and survival of GCB DLBCL cells in the absence of serum. PKK may play a crucial role in the survival of DLBCL cells by mediating NF-κB activation induced by other signaling molecules in addition to BAFF. Additional studies are needed to address these issues. It was previously reported that overexpression of PKK can activate NF-κB reporter in an IKKγ (NEMO)–independent manner.38 It would thus be interesting to investigate whether IKKγ is required for BAFF-induced NF-κB activation in DLBCL cells in future studies. Further molecular understanding of PKK function will not only provide new insights into BAFF signaling but also into the mechanism underlying development of certain B cell–related diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Hartmut Land, Andrea Bottaro, and Daniel Ryan for helpful discussions and critical reading of this manuscript. We also thank Warren Pear for pMIG vector, Gary Nolan for the amphotropic viral packaging cell line, Edward Schwarz for the GST-IκBα expression plasmid, John Ashton for the NF-κB luciferase reporter, and Timothy Bushnell for help with flow cytometric analysis.
This work was supported in part by the Edelman-Gardner Foundation and the James P. Wilmot Cancer Center of the University of Rochester.
Authorship
Contribution: S.-W.K. designed some of the experiments, performed research, analyzed data, and participated in writing the paper; D.W.O. performed research and analyzed data; R.M.R. performed in vivo tumor growth experiments and analyzed data; C.T.J. provided reagents, analyzed data, and critically reviewed the paper; I.S. provided scientific support, analyzed data, and critically reviewed the paper; L.C. designed and performed research, analyzed data, and wrote the paper; J.Z. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jiyong Zhao, Department of Biomedical Genetics, University of Rochester, 601 Elmwood Ave, Rochester, NY14642; e-mail: jiyong_zhao@urmc.rochester.edu; or Luojing Chen, Division of Allergy/Immunology and Rheumatology, University of Rochester, 601 Elmwood Ave, Rochester, NY 14642; e-mail: luojing_chen@urmc.rochester.edu.