To the editor:
The US approach to optimizing medical preparedness to a mass nuclear irradiation disaster has been nicely outlined in Weinstock et al,1 and we thought it might be of interest, and relevance, to present in more depth the European approach referred to in the article.
In March 2002, the European Group for Blood and Marrow Transplantation (EBMT) established a Nuclear Accident Committee (NAC) to determine whether the EBMT resource of more than 500 hospitals could be optimized as a network for providing help in the event of a radiation disaster.2
In 2005, an EBMT International Consensus Meeting defined a unified basis for the medical management of radiation accident victims.3 The core of this consensus was the 2001 “METREPOL” (Medical Treatment Protocols for Radiation Accident) clinical grading of irradiated victims, based on data from 800 victims in 70 previous accidents.4 METREPOL uses assessment of hematologic (H), neurovascular (N), cutaneous (C), and gastrointestinal (G) damage early after exposure to predict outcome.4,5 It incorporates simple clinical laboratory tests such as haematologic blood counts, and, particularly, identifies the likelihood of “irreversible” (H4), and “reversible” (H3, H2, and H1) damage to the bone marrow.6,7 METREPOL links the 4 systems (H, N, C, G) together, to identify early after exposure the potential for developing multiorgan failure.8
The US approach for predicting outcome is different and uses biodosimetry (chromosome changes in blood lymphocytes) as the driving force for deciding the outcome of a patient. However, in an emergency involving, say, 30 000 patients with myeloid suppression, the use of chromosome analysis to estimate a dose would be impossible simply due to logistic reasons.1 With the “METREPOL” approach, the combination of clinical parameters and simple blood count changes would be logistically feasible.
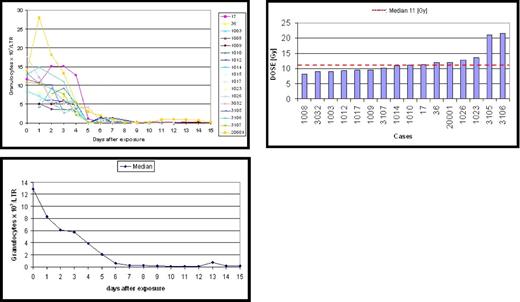
In addition, we feel METREPOL may be a more accurate predictor of outcome. In the group of irradiated patients illustrated in Figure 1, there is a uniform pattern seen in blood parameters after irradiation, with an initial granulocytosis, followed by a drop by day 7. This identifies a homogeneous group of patients with irreversible damage to their stem cell pool (H4) such that they will need SCT rescue if they are to have a chance of survival.6 However, the calculated biodosimetry in these same patients showed heterogeneity, with a wide estimated dose range from 8 to 20 Gy.
Granulocyte changes in 17 patients who are characteristic for a grading code H4 observed in 6 radiation accidents caused by whole body exposure to pure gamma or a mixed neutron/gamma radiation. An irreversible injury to the bone marrow stem cell pool can be assumed if the pattern of granulocyte concentration changes shows a severe granulocytopenia on days 5/6 (grade H4). However, the “dose” was reported to range from 8 to 20 Gy (median 11 Gy). A computerized program is under development to assess blood cell changes early after exposure and calculate the assignment of a patient to a grade H. The cases are documented in the database system SEARCH5 and have been analyzed scientifically.4,6-8 This figure was presented at the RITN meeting on September 25, 2007, in Bethesda, MD.
Granulocyte changes in 17 patients who are characteristic for a grading code H4 observed in 6 radiation accidents caused by whole body exposure to pure gamma or a mixed neutron/gamma radiation. An irreversible injury to the bone marrow stem cell pool can be assumed if the pattern of granulocyte concentration changes shows a severe granulocytopenia on days 5/6 (grade H4). However, the “dose” was reported to range from 8 to 20 Gy (median 11 Gy). A computerized program is under development to assess blood cell changes early after exposure and calculate the assignment of a patient to a grade H. The cases are documented in the database system SEARCH5 and have been analyzed scientifically.4,6-8 This figure was presented at the RITN meeting on September 25, 2007, in Bethesda, MD.
For triage after a mass radiation event, we feel that prehospital triage should take place, based on symptoms and the location of the patient during radiation exposure, to prevent blockade of hospitals. A second triage occurs at the referred hospital after decontamination.
Lastly, in the Weinstock paper there is little about training or communications. EBMT training started in November 2007 in Munich. We also have a network of electronic communications in place, a key to optimizing allocation of irradiated patients to specialized hospitals. This facilitates real-time data collection, which not only allows for the best care but also is a research resource for future incidents and for optimizing METREPOL.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests. Ray Powles sits on the advisory boards of pharmaceutical companies Schering-Plough, Hakko-Kyowa, and LipoNova.
Correspondence: Theodor M. Fliedner, Radiation Medicine Research Group, Ulm University, Helmholtzstrasse 20, Ulm, Germany 89081; e-mail: theodor.fliedner@uni-ulm.de.