Abstract
Rituximab, an anti-CD20 monoclonal antibody, has been used to treat autoimmune disorders such as mixed cryoglobulinemia (MC). However, its mechanisms of action as well as the effects on cellular immunity remain poorly defined. We investigated the changes of peripheral blood B- and T-cell subsets, the clonal VH1–69 cells, as well as the cytokine profile following rituximab therapy. The study involved 21 patients with hepatitis C–related MC who received rituximab, of whom 14 achieved a complete response. Compared with healthy and hepatitis C virus (HCV) controls, pretreatment abnormalities in MC patients included a decreased percentage of naive B cells (P < .05) and CD4+CD25+FoxP3+ regulatory T cells (P = .02) with an increase in memory B cells (P = .03) and plasmablasts (P < .05). These abnormalities were reverted at 12 months after rituximab. Clonal VH1–69+ B cells dramatically decreased following treatment (32% ± 6% versus 8% ± 2%, P = .01). Complete responders of rituximab exhibited an expansion of regulatory T cells (P < .01) accompanied with a decrease in CD8+ T-cell activation (P < .01) and decreased production of interleukin 12 (IL-12; P = .02) and interferon-γ (IFN-γ; P = .01). Our findings indicate that in patients with MC, response to B-cell depletion induced by rituximab effectively normalizes many of the disturbances in peripheral B- and T-lymphocyte homeostasis.
Introduction
Chronic infection with hepatitis C virus (HCV) is the main cause of mixed cryoglobulinemia (MC) vasculitis, a potentially life-threatening, systemic vasculitis.1 MC reflects the expansion of B cells producing a pathogenic IgM with rheumatoid factor (RF) activity. MC may lead to clinical manifestations ranging from an MC syndrome (purpura, arthralgia, asthenia) to a more serious vasculitis with neurologic and renal involvement.2 Oligoclonal or monoclonal B-cell expansions are significant molecular features of MC.3,4 It is estimated that almost 10% of patients with MC progress to frank B-cell non-Hodgkin lymphoma (NHL). The overall risk of NHL in patients with HCV-MC is estimated to be 35 times higher than in the general population.5
HCV is capable of infecting B-lymphocytes through LDL receptors or CD81.6,7 The E2 envelope glycoprotein of HCV can bind CD81 B-cell surface protein.6 CD81 has been demonstrated to play a costimulatory role in T-cell activation and an inhibitory role on natural killer (NK) cell on binding to E2 glycoprotein.8,9 CD81 on B-cell surface may provide a strong stimulatory signal if activated as a part of a complex (CD19/CD21/CD81 complex) together with BCR activation.10 The ability of E2 to directly engage CD81 alongside the binding of specific anti-E2 BCR may create a powerful stimulatory signal, promoting proliferation. Clonal B-cell expansion has been demonstrated in liver, blood, and bone marrow of patients with chronic HCV infection in the absence of overt B-cell malignancy.3,4 The B-cell repertoire in cryoglobulinemic vasculitis is highly restricted. B-cell clones in HCV-MC often use the VH1-69 gene and VkA27 segment, which is also used by anti-E2 antibodies elicited by HCV.11,12 Sequencing of these Ig variable regions has also revealed that they are the product of somatic hypermutation.13 In addition, recent evidence suggests a role in MC for high serum levels of B lymphocyte stimulator (BLys), a member of the tumor necrosis factor family of cytokines that promotes B-cell maturation and survival and plasma cell differentiation.14,15
The pathogenesis of HCV-MC vasculitis is complex and is likely to involve many mechanisms. Neutrophilic infiltration with leukocytoclastic changes, typical of immune complex–mediated vasculitis, has seldom been found, while the presence of lymphohistiocytic infiltrates suggests a T cell–mediated pathogenesis.16-19 Patients with HCV-MC vasculitis display a disturbed peripheral blood T-cell repertoire.20 CD4+CD25+ regulatory T cells, which have been shown to control autoimmunity, are significantly reduced in HCV-MC vasculitis patients.20 Further evidence that T cells help in the production of cryoglobulins is the influence of human leukocyte antigen (HLA) type II polymorphism on the production of HCV-related cryoglobulins.21
Anti-CD20 monoclonal antibody (rituximab) proved to be very effective in patients with HCV-MC.22-30 A complete clinical response was achieved in 60% to 70% of cases.22-29 However, little evidence is available about immunologic dynamics after rituximab-mediated depletion. In autoimmune diseases, B cells may disturb immune homeostasis by multiple mechanisms in addition to the production of pathogenic autoantibodies, including autoantigen presentation, cytokine production, and modulation of the T-cell activation and T-cell differentiation.31-33 As a first examination of the immunologic effects of rituximab in MC vasculitis, we evaluated both B- and T-cell subsets in patients at baseline, in the setting of selective B-cell depletion by anti-CD20 monoclonal antibodies, and during the B-cell recovery phase. This is the first mechanistic study of the effects of B-cell depletion on cellular abnormalities in patients with HCV-MC vasculitis. Our results indicate that targeted B-cell depletion effectively normalizes many of the disturbances in peripheral B- and T-lymphocyte homeostasis.
Methods
Patients
Approval was obtained from the Pitie-Salpetriere hospital (Paris, France) institutional review board for this study, and informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Twenty-one patients with HCV-MC vasculitis (mean age, 53.5 years; range, 24-81 years) entered the study. All had positive HCV antibodies and RNA and were negative for hepatitis B surface antigen and anti-HIV antibodies. MC patients had a serum cryoglobulin level of more than 0.05 g/L, at least at 2 determinations. Serum cryoglobulin level was measured as described elsewhere.34 Laboratory evaluation included rheumatoid factor, immunofixation, IgM level, and C4 fraction of complement. The mean plus or minus SD levels of serum cryoglobulin, C4 complement factor, and rheumatoid factor were 0.71 plus or minus 0.3 g/L, 0.04 plus or minus 0.06 g/L, and 194 plus or minus 225 IU/L, respectively. Nineteen patients (90.5%) had a monoclonal IgM kappa. MC vasculitis was defined by the presence of serum cryoglobulin associated with the triad of purpura-arthralgia-asthenia with renal and/or neurologic involvement. Clinical manifestations of MC vasculitis included purpura (n = 17), arthralgia (n = 13), peripheral neuropathy (n = 12), renal involvement (n = 9), and leg ulcer (n = 3). Patients receiving antiviral or immunosuppressive treatment in the 3 months preceding inclusion were excluded. Controls include 50 healthy blood donors (mean age, 44.6 years; range, 26-73 years) and 20 chronically infected HCV patients without serum cryoglobulin and vasculitis (mean age, 49.2 years; range, 29-77 years).
Treatment
Twenty-one patients with MC vasculitis were treated with rituximab. The therapeutic schedule consisted of the weekly administration of 4 intravenous infusions of rituximab at 375 mg/m2 (on days +1, +8, +15, and +22) over a period of 1 month. Premedication with methylprednisolone 40 mg intravenously was given before each infusion of rituximab.
Clinical response of MC vasculitis was defined by analyzing the evolution of the following main clinical signs: skin involvement (absence of purpura), peripheral neuropathy (clinical and electrophysiologic improvement on 2 successive examinations), renal involvement (normalization of serum creatinine level and disappearance of proteinuria), and the absence of arthralgia. A complete clinical response was defined as a full improvement in all baseline clinical manifestations. A partial clinical response was defined as improvement in at least one-half of the baseline clinical manifestations. Patients who had neither a complete clinical response nor a partial clinical response were classified as nonresponders. Samples were collected at baseline and at 1, 3, 6, 9, and 12 months following rituximab infusions.
Flow cytometry
The concentration (cells/μL) of lymphocytes, CD4+, CD8+ T lymphocytes, and CD19+ B lymphocytes was established from fresh blood samples with a 4-color flow cytometry analysis multitest with Trucount tubes containing fluorescents beads as internal standard, according to the manufacturer's instructions (BD Biosciences, Mountain View, CA).
Peripheral blood mononuclear cells (PBMCs) were isolated from blood sample using Ficoll-Hypaque density gradient centrifugation. PBMCs were washed twice in PBS containing 2% fetal calf serum (FCS) and numerated before labeling. The flow cytometric analysis of lymphocytes subpopulations was performed using a panel of monoclonal antibodies conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP), phycoerythrin-cyanyn 5(PE-Cya5), or allophycocyanine (APC) to the following cell surface proteins: CD3, CD4, CD19, CD20, CD8, CD45RA, CD45RO, glucocorticoid-induced tumor necrosis factor receptor (GITR), and CCR7 (all from Beckman Coulter, Villepinte, France), CD4, CD25, CD27, CD38, CD62L, HLA-DR, and IgD were from BD Biosciences.
Irrelevant antibodies of respective isotypes were used as negative controls. Briefly, cells were incubated with the appropriate antibodies at 4°C for 20 minutes, washed in PBS containing 2% of FCS, and then fixed in PBS containing 1% paraformaldehyde.
Intranuclear forkhead box P3 (FoxP3) labeling was performed after CD3, CD4, and CD25 membrane staining using APC antihuman Foxp3 kit (PCH101 clone; eBiosciences, San Diego, CA) according to the manufacturer's instructions. Rat IGg2a APC was used as isotypic control (eBiosciences).
The mAb G6 was provided by Dr R. Jefferis (University of Birmingham, Birmingham, United Kingdom). G6 reacts with an epitope of the VH1-69 VH gene product.35
Cells acquisition and analysis by flow cytometry were performed using a FACScalibur (Becton Dickinson, Lincoln Park, NJ) or a FC500 Cytometer (Beckman Coulter). Instrument setting parameters (gains, compensations, and threshold) were set with machine software (CXP Software; Beckman Coulter) in conjunction with calibration beads (Flow-set beads, Cytocomp kit, and CYTO-TROL Control Cells). Machine reproducibility was verified with standardized beads (Flow-check).
Cytokines were quantified in patients' sera using a Multiplex Fluorescent Bead Immunoassay FlowCytomix human Th1/Th2 11plex (Bender, Vienna, Austria) with a FC500 Cytometer (Beckman Coulter).
Statistical analysis
Analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA). Data were expressed as mean plus or minus SEM. Student t test for independent samples was used for comparisons between different groups and the paired sample t test for within-group comparisons. A 2-sided P value less than .05 was considered statistically significant.
Results
The peripheral blood phenotype of B (naive, pre–germinal center, memory, marginal zone, plasmablast, and clonal VH1-69 cells) and T (activated, naive, memory, and regulatory) cells was analyzed in patients with HCV-MC vasculitis (n = 21) and compared with healthy controls (n = 15) and HCV controls without serum cryoglobulin and vasculitis (n = 20). The cytokine profile of HCV-MC patients was also studied. Results were analyzed before and after rituximab therapy and in relation to clinical response of MC vasculitis.
Abnormalities in peripheral cell homeostasis of HCV-MC vasculitis
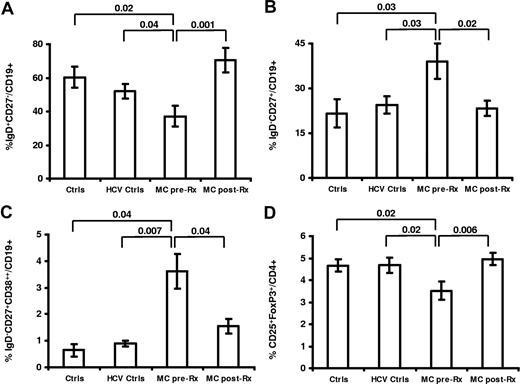
Peripheral blood B cells were initially examined for the expression of CD27, IgD, and CD38 to differentiate naive (IgD+CD27−CD38low), memory (IgD−CD27+CD38low), pre-GC (IgD+CD27−CD38high), marginal zone (IgD+CD27+), and plasmablast (IgD−CD27highCD38high) B cells.36 Using this approach, we identified a naive B lymphopenia with an increase in memory and plasmablast cells (Figure 1). We detected a reduction of naive B cells in the peripheral blood of patients with MC vasculitis before treatment with rituximab (mean ± SEM: 37.2% ± 6.1%) compared with healthy (60.4% ± 6.3%, P = .02) and HCV controls (52.1% ± 4.3%, P = .03; Figures 1, 2). MC vasculitis patients displayed a significant expansion of peripheral blood memory B cells and plasmablasts at baseline compared with healthy and HCV controls (memory: 39.0% ± 5.9% vs 21.6% ± 4.7% [P = .03] vs 24.5% ± 2.9% [P = .03]; plasmablasts: 3.6% ± 0.6% vs 0.6% ± 0.2% vs 0.9% ± 0.1% [P = .007]; Figures 1, 2). The levels of CD20 expression in the population of naive, memory, pre-GC, MZ, and plasmablast B cells were 99% ± 0.9%, 94% ± 4.3%, 99% ± 0.7%, 94% ± 5.2%, and 14% ± 14.4%, respectively. No significant change in the CD20 expression was observed after rituximab therapy except a trend toward a lower CD20 expression in the memory B-cell population (94% ± 4.3% to 76% ± 7.2%).
Abnormalities in peripheral cell homeostasis of HCV-MC vasculitis revert following rituximab. Naive (IgD+CD27−CD38low) lymphopenia (A), expansion of memory (IgD−CD27+CD38low) B cells (B) and plasmablasts (IgD−CD27highCD38high) (C), and quantitative deficiency of regulatory T cells CD4+CD25+FoxP3+ (D) in patients with mixed cryoglobulinemia (MC) vasculitis (MC pre-Rx) (n = 21) compared with healthy volunteers (Ctrls) (n = 15) and chronic HCV patients without MC-vasculitis (HCV Ctrls) (n = 20). Improvement of peripheral B- and T-cell abnormalities after rituximab in patients with MC vasculitis (MC post-Rx). Data are expressed as mean plus or minus SEM.
Abnormalities in peripheral cell homeostasis of HCV-MC vasculitis revert following rituximab. Naive (IgD+CD27−CD38low) lymphopenia (A), expansion of memory (IgD−CD27+CD38low) B cells (B) and plasmablasts (IgD−CD27highCD38high) (C), and quantitative deficiency of regulatory T cells CD4+CD25+FoxP3+ (D) in patients with mixed cryoglobulinemia (MC) vasculitis (MC pre-Rx) (n = 21) compared with healthy volunteers (Ctrls) (n = 15) and chronic HCV patients without MC-vasculitis (HCV Ctrls) (n = 20). Improvement of peripheral B- and T-cell abnormalities after rituximab in patients with MC vasculitis (MC post-Rx). Data are expressed as mean plus or minus SEM.
Flow cytometry figure comparing B-cell populations in a healthy volunteer (Ctrl), and in a patient with HCV-MC vasculitis before (MC pre-Rx) and after (at month +1 [MC post-Rx M1] and at month +12 [MC post-Rx M12]) rituximab.
Flow cytometry figure comparing B-cell populations in a healthy volunteer (Ctrl), and in a patient with HCV-MC vasculitis before (MC pre-Rx) and after (at month +1 [MC post-Rx M1] and at month +12 [MC post-Rx M12]) rituximab.
Peripheral blood CD4+ and CD8+ T cells were examined for the expression of HLA-DR (activated), CD45RA (naive), CD45RO (memory), and CD25+FoxP3+ (regulatory). As previously described with CD4+CD25++,20 we identified a decreased frequency of CD4+CD25+FoxP3+ regulatory T cells in the peripheral blood of patients with MC vasculitis before treatment with rituximab compared with healthy donors (3.5% ± 0.8% vs 4.6% ± 0.6%, P = .02) and HCV controls (3.5% ± 0.8% vs 4.6% ± 0.5%, P = .03).
Rituximab improves peripheral cell homeostasis in HCV-MC vasculitis
After treatment with rituximab and immune reconstitution (≥ 12 months after treatment), there was a statistically significant increase in the percentage of naive B cells in patients with MC vasculitis (37.2% ± 6.1% vs 64.3% ± 5.0%, P = .001; Figure 1). The IgD−CD27+ memory and IgD−CD27+CD38+++ plasmablast B-cell expansion decreased significantly (38.9% ± 5.9% vs 23.3% ± 2.6% [P = .02] and 3.3% ± 0.7% vs 1.6% ± 0.2% [P = .04], respectively; Figure 1).
In the peripheral T-cell population, a significant increase in the percentage of CD4+CD25++FoxP3+ regulatory T cells was also evidenced after treatment with rituximab (3.5% ± 0.4% vs 4.9% ± 0.2%, P = .006; Figure 1).
B-cell depletion (< 1% of the total peripheral blood lymphocytes) was achieved in 67% (14/21) of the MC vasculitis group regardless of the clinical response. Even in patients with effective B-cell depletion to fewer than 5 cells/μL (to > 99% reduction), residual CD19+ B cells could be detected in the peripheral blood at the time point of maximal depletion, prior to immune reconstitution (Figure 3). The remaining B cells were predominantly of switched memory phenotype (CD19+, CD38low, CD27+, IgD−; P < .01) with a smaller percentage representing IgD+CD27+ marginal zone cells (P < .05) (Figures 2, 3). The percentage of CD19+ cells (12.7% ± 2.2%) dropped to less than 1% after the fourth infusion. Recovery of B-cell count began from 6 to 9 months (Figure 3). Serum cryoglobulin titer changed significantly by 1 year after treatment (0.71 ± 0.3 g/L to 0.18 ± 0.1 g/L, P < .01) (Figure 3). Complete responders of MC vasculitis exhibited a higher decrease in the cryoglobulin titer after rituximab compared with partial or nonresponders (decrease of 86.5% of pretreatment values vs 56.7%, respectively; P < .05). Rheumatoid factor and IgM levels decreased by 1 year after treatment from 194 ± 225 IU/L to 53 ± 86 IU/L (P < .01) and 1.77 ± 0.82 g/L to 1.31 ± 0.63 g/L (P = .26), respectively. The C4 serum complement level increase significantly by 1 year after treatment from 0.04 plus or minus 0.06 g/L to 0.14 plus or minus 0.11 g/L (P < .01).
Effects of B-cell depletion on peripheral B-cell populations and serum cryoglobulin. (A) Histogram representing the percentage of CD19+ cells following rituximab in patients with HCV-MC vasculitis. (B) Residual B-cell populations after rituximab-mediated depletion (*P < .05, **P < .01). (C) Serum cryoglobulin level (g/L) during rituximab therapy in patients with HCV-MC vasculitis (**P < .01).
Effects of B-cell depletion on peripheral B-cell populations and serum cryoglobulin. (A) Histogram representing the percentage of CD19+ cells following rituximab in patients with HCV-MC vasculitis. (B) Residual B-cell populations after rituximab-mediated depletion (*P < .05, **P < .01). (C) Serum cryoglobulin level (g/L) during rituximab therapy in patients with HCV-MC vasculitis (**P < .01).
Effects of rituximab on Vh1-69 clonal B cells
We next asked whether rituximab treatment alters Vh1-69 clonal B cells production.
Vh1-69 heavy chain rearrangement was identified in high frequency among patients with HCV-induced MC vasculitis and lymphoproliferation.37 It is thought that Vh1-69–positive cells may represent B cells that are trying to mount an immunoglobulin response against the E2 viral envelope protein and commonly undergo clonal proliferation.37 We have studied Vh1-69 rearrangement with a specific antibody directed against the Vh1-69 gene product (G6). Vh1-69–positive samples (G6+ cells > 5% of CD19+ cells) were found in 6 of 21 HCV-induced MC vasculitis patients, accounting for 10% to 91% of the entire CD19+ populations (mean ± SEM: 32% ± 6%). No significant proportion of G6+ cells was found in healthy controls (n = 4) or HCV+ patients without MC-vasculitis (n = 9). G6+ cells were characterized by a CD27+IgD−CD38low or CD27+IgD+CD38low staining (Figure 4). Following rituximab, the Vh1-69–positive cell proportion in HCV-MC vasculitis patients decreased to 8% ± 2% (P = .01) of the entire CD19+ populations (Figure 4).
Effects of rituximab on VH1-69 clonal B cells. (A) Flow cytometry figures of a patient with HCV-MC vasculitis demonstrating staining with anti–Vh1-69 gene product mAb (MC pre-Rx) and disappearance of VH1-69+ B cells following rituximab (MC post-Rx). (B) Histogram representing the percentage of VH1-69+ cells among CD19+ B cells (mean ± SEM) in patients with HCV-MC vasculitis (n = 11) before (MC pre-Rx) and after (MC post-Rx) rituximab.
Effects of rituximab on VH1-69 clonal B cells. (A) Flow cytometry figures of a patient with HCV-MC vasculitis demonstrating staining with anti–Vh1-69 gene product mAb (MC pre-Rx) and disappearance of VH1-69+ B cells following rituximab (MC post-Rx). (B) Histogram representing the percentage of VH1-69+ cells among CD19+ B cells (mean ± SEM) in patients with HCV-MC vasculitis (n = 11) before (MC pre-Rx) and after (MC post-Rx) rituximab.
B-cell depletion–induced immunologic responses in relation to clinical outcome
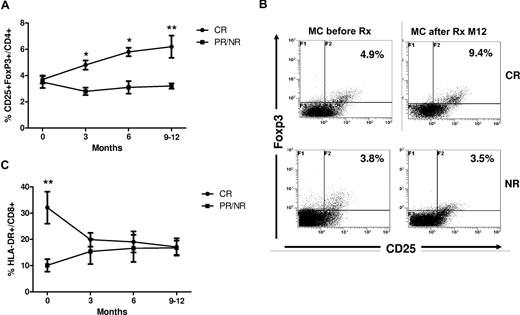
We next analyzed whether patients who achieved complete clinical response (CR; n = 14; 66.6%) displayed a distinct immunologic response to rituximab compared with those who achieved a partial response (PR; n = 6; 28.6%) or nonresponse (NR; n = 1; 4.8%; Figure 5; Table 1).
B-cell depletion induced immunologic responses in relation to clinical outcome in patients with HCV-MC vasculitis. (A) The percentage of CD4+CD25+FoxP3+ regulatory T cells increased significantly in HCV-MC patients with complete clinical response (CR; n = 14; circles) following rituximab compared with patients with partial response and nonresponse (PR/NR; n = 7; squares; *P < .05, **P < .01). (B) Flow cytometry figures showing the increase in CD4+CD25+FoxP3+ regulatory T cells following rituximab (at month + 12) in a patient with complete response (CR) of HCV-MC compared with nonresponse (NR). (C) The percentage of CD8+HLA-DR+ activated T cells normalized in HCV-MC patients with complete clinical response (CR; circles) following rituximab compared with those with partial response and nonresponse (PR/NR; n = 7; squares; **P < .01). Data are expressed as mean plus or minus SEM.
B-cell depletion induced immunologic responses in relation to clinical outcome in patients with HCV-MC vasculitis. (A) The percentage of CD4+CD25+FoxP3+ regulatory T cells increased significantly in HCV-MC patients with complete clinical response (CR; n = 14; circles) following rituximab compared with patients with partial response and nonresponse (PR/NR; n = 7; squares; *P < .05, **P < .01). (B) Flow cytometry figures showing the increase in CD4+CD25+FoxP3+ regulatory T cells following rituximab (at month + 12) in a patient with complete response (CR) of HCV-MC compared with nonresponse (NR). (C) The percentage of CD8+HLA-DR+ activated T cells normalized in HCV-MC patients with complete clinical response (CR; circles) following rituximab compared with those with partial response and nonresponse (PR/NR; n = 7; squares; **P < .01). Data are expressed as mean plus or minus SEM.
We identified 2 main differences in T-cell response in relation to clinical outcome. First, the percentage of CD4+CD25++FoxP3+ regulatory T cells was significantly increased after rituximab in patients with CR of MC vasculitis compared with before treatment levels (3.7% ± 0.3% at baseline vs 6.2% ± 0.8% at month 9/12, P < .01; Figure 5). In contrast, in MC patients with PR/NR the frequency of CD4+CD25+FoxP3+ cells remained unchanged following rituximab (3.5% ± 0.4% at baseline vs 3.2 ± 0.2% at month 9/12, P = .8). The regulatory T-cell percentage in patients with CR became approximately 2-fold higher compared those with PR/NR after rituximab therapy (at month 9/12, 6.2% ± 0.8% vs 3.2% ± 0.2%, respectively, P < .01). CD4+CD25+FoxP3+ phenotyping was performed with CD45RA, CD45RO, GITR, CD62L, and CCR7 staining to assess possible treatment-related changes in regulatory T-cell characteristics. CD4+CD25+FoxP3+ cells were characterized by a CD45RO+CD45RA−CD62L+GITR+CCR7low phenotype. We did not find significant changes in this phenotype during or after rituximab therapy, regardless of treatment response (not shown).
In addition, a decrease in T-cell activation accompanies clinical remission and regulatory T-cell expansion following rituximab. The percentage of activated CD8+DR+ cells at baseline was higher in MC patients with CR compared with those with PR/NR (32.1% ± 6.1% vs 10.1% ± 2.4%, respectively, P < .01). After treatment with rituximab, the CD8+DR+ activated T-cell expansion improved significantly in patients with CR (32.1% ± 6.1% at baseline vs 17.1% ± 3.3% at month 9/12, P < .01), whereas no significant change was noted in those with PR/NR (10.1% ± 2.4% at baseline vs 16.8% ± 2.7% at month 9/12, P = .9; Figure 5).
Finally, a striking change observed following completion of rituximab treatment was that the Th1 polarization in MC vasculitis patients can be reverted in CR (Table 1). Interleukin-12 (IL-12) production decreased significantly in patients with CR compared with patients with NR/PR (decrease of 81.5% of pretreatment values vs 39.2%, respectively, P < .05). Another Th1 cytokine, interferon-γ (IFN-γ), decreased significantly in patients with CR compared with patients with NR/PR (decrease of 88.7% of pretreatment values vs 44.9%, respectively, P < .05; Table 1).
Discussion
The pathogenesis of MC vasculitis has traditionally been considered to be immune complex mediated. However, a number of T-cell abnormalities have been described, including a polarization toward Th1 subsets17,19,38 and also a quantitative deficiency of circulating regulatory T cells.20 B-cell depletion has been used in the treatment of patients with a number of different autoimmune diseases. Rituximab proved to be very effective in patients with HCV-MC22-30 with clinical improvement in up to 60% of cases.22-29 Although favorable outcomes have been reported with rituximab, little evidence is available about clinical and immunologic dynamics. Given the unclear nature of B-cell depletion, the multiple actions of B-cell regulation of T cells, and the unclear mechanism of autoimmunity in MC, we aimed to explore the immunologic dynamics in MC patients being treated with rituximab.
The results of our study demonstrate that CD20-targeted B-cell depletion in the treatment of MC vasculitis effectively normalizes the significant disturbances in peripheral B-lymphocyte homeostasis. We identified significant B-cell abnormalities in patients with MC vasculitis compared with healthy and HCV controls—notably, a striking naive B-cell lymphopenia and a marked expansion of memory B cells and plasmablast. In addition, MC vasculitis is characterized by a marked increase in circulating VH1-69 clonal B cells that is dramatically reduced by rituximab therapy. The pool of CD27+ peripheral B cells is less susceptible to immunosuppressive therapy in contrast to the pools of naive B cells and plasmablast cells. These features are reminiscent of that observed in systemic lupus erythematosus.39,40 Consistent with this normalization of B-cell homeostasis, we observed a significant decrease in serum cryoglobulin level, the hallmark of MC-vasculitis, and a clinical improvement in the majority of patients. Complete responders of MC vasculitis exhibited a 30% higher decrease in the cryoglobulin titer after rituximab compared with partial or nonresponders.
In our study, we found variability in B-cell depletion. Multiple factors likely underlie this variability, including differences in rituximab pharmacokinetics and Fc receptor polymorphisms that directly impact effector mechanisms of B-cell killing mediated by the anti-CD20 monoclonal antibody. However, disease activity was not associated with impaired B-cell depletion. Alternatively, B-cell subsets that are preferentially expanded in MC vasculitis, such as CD19+CD38lowCD27+IgD− memory cells or to a lesser extent CD27+IgD+ marginal zone cells, might be less susceptible to rituximab, accounting for the ineffective depletion observed in some patients.
Ideally, B-cell depletion provides the immune system with a new chance for proper regulation of emerging autoreactive B lymphocytes and restoration of tolerance. The precise nature and appropriate measure of B-cell tolerance in MC patients remain poorly understood. As highlighted by the variability in serum cryoglobulin response in our study, measurement of autoantibodies represents only an indirect marker that could also reflect the persistence of plasma cells with a heterogeneous lifespan. We suggest that determining the fate of clonal VH1-69 B cells represents an additional and powerful biomarker of tolerance in MC patients who rearrange this Ig gene. Disappearance of clonal VH1-69 memory B cells in MC patients treated with rituximab may reflect restoration of a B-cell tolerance checkpoint at the level of the germinal center.
Given the T-cell abnormalities observed in patients with MC,19,20 it has not been clear how B-cell depletion works in MC vasculitis. Although tempting to suggest the response is related to depletion of the antibody-producing B cells, a number of points suggest it may be more complicated, such as the global persistence of the pool of memory B cells and the fact that response often occurs while serum cryoglobulin can still be detectable. Our results indicate that many T-cell abnormalities found in MC can be reverted after treatment with rituximab in relation with complete clinical response. Regulatory T cells, which have been shown to control autoimmunity and are significantly reduced in HCV-MC patients,20 dramatically expanded in CR following rituximab. Along this line, a decrease in T-cell activation accompanies clinical remission and regulatory T-cell expansion following rituximab. The activated CD8+ T-cell expansion normalized after anti-CD20 monoclonal antibodies in CR. Recent investigations have shown that Th1/Th2 imbalance plays a central role in HCV-MC vasculitis.19,38 It is important to emphasize that several factors can determine the fate of activated T cells, including type of antigen-presenting cells, costimulatory molecules, chromatin structure, and cytokines present in the local microenvironment of the cell at the time of stimulation. Interestingly, the data present here indicate that Th1 polarization can be reverted after treatment with rituximab, but only in complete responders of MC vasculitis. Limited evidence from the literature supports the view that rituximab affects both the cellular and the humoral arm of the immune system. Vallerskog et al studied 11 patients with systemic lupus erythematosus.41 They observed that upon B-cell repopulation there was a significant increase in CD4+CD25+FoxP3+ regulatory T cells.41 Sfikakis et al reported a significant decrease of the costimulatory molecule CD40 ligand on CD4+ T cells and in the expression of T-cell activation markers CD69 and HLA-DR associated with clinical remission of lupus nephritis following B-cell depletion.42 Cross et al demonstrated that rituximab reduces both B cells and T cells in the cerebrospinal fluid of multiple sclerosis patients.43 Stasi et al showed the reversion of T-cell abnormalities (ie, decrease of the Th1/Th2 ratio, decreased expression of Fas ligand on Th1 and Th2 cells, decreased expression of Bcl-2 mRNA, increased expression of Bax mRNA, and the reversion of the oligoclonal T-cell repertoire) in patients with idiopathic thrombocytopenic purpura who responded to rituximab therapy.44 Of particular interest in this context is the 2-way interaction between B cells and T cells. B cells provide signals to T cells through antigen presentation, and T cells provide “help” to B cells through the delivery of cytokines and cell-surface ligands. These interactions create the potential for a positive-feedback loop or “vicious cycle.”
In conclusion, this is the first study to show that in HCV-MC vasculitis, B-cell depletion therapy with rituximab dramatically improves abnormalities in B-cell homeostasis, with a decreased proportion of autoreactive memory B cells after treatment. In addition, the results reported herein indicate that B-cell depletion may be an efficient therapy not only because it reduces or abolishes the hyperproduction of cryoglobulin, but also because this treatment improves T-cell homeostasis in restoring the regulation/activation and Th1/Th2 imbalances.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Dr P. Ghillani-Dalbin for help with the immunobiologic data. We are also extremely grateful to Veronique Bon Durand, Cornelia Degbe, Vanessa Godie, and Nathalie Fery for their technical assistance.
This work was supported by Agence Nationale de Recherche contre le SIDA, Centre National de la Recherche Scientifique, Faculté de Médecine Pitié-Salpêtrière, and Assistance Publique-Hôpitaux de Paris.
Authorship
Contribution: D.S., M.R., J.C.P., D.K., and P.C. designed research; D.S., D.L., and M.R. performed research; D.S., M.R., and P.C. analyzed the data; D.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patrice Cacoub, Service de Médecine Interne, Groupe Hospitalier La Pitié-Salpêtrière, 47-83, Boulevard de l'Hôpital, 75013 Paris, France; e-mail: patrice.cacoub@psl.aphp.fr; or David Klatzmann, Service de Biothérapie, Groupe Hospitalier La Pitié-Salpêtrière, 47-83, Boulevard de l'Hôpital, 75013 Paris, France; e-mail: david.klatzmann@chups.jussieu.fr.
References
Author notes
D.S. and M.R. contributed equally to this work.
D.K. and P.C. should be considered co-senior authors.

![Figure 2. Flow cytometry figure comparing B-cell populations in a healthy volunteer (Ctrl), and in a patient with HCV-MC vasculitis before (MC pre-Rx) and after (at month +1 [MC post-Rx M1] and at month +12 [MC post-Rx M12]) rituximab.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/11/10.1182_blood-2007-11-122713/6/m_zh80090818790002.jpeg?Expires=1769084789&Signature=h8MKejUv6r-aQqLq8yAr5cxbUN3MxSp2g4nHc~VQOx-5DbdCMNMvN4JqFXb9qPvLWEZTRVf8Ki3PT2ff~c3ffryUXaybOzH5f0zpobAeAxDDVElcOMXNvFfj0zrbIfk8Jwq1AmmZTPWPvMWD0rsqO3SbkIoXtDTmpCQSGkvDBuoFwYbzJmEmdVZDaX-9tzHcR3QiN9B0r~OWV7TAjmD6MfynWhjGzmWY4~rhs0RnaLVY1yfjhtbm0qEHfefwI9jmtjkOA6HRslzyWBGpcWC4ZLdnrlaoLLafiwjf7GNypGMEiYhGNbN7ofD5EVBw2---y7QKuSCchM63GHDdMaAbQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)