Abstract
Although leukemic stem cells (LSCs) show a symbiotic relationship with bone marrow microenvironmental niches, the mechanism by which the marrow microenvironment contributes to self-renewal and proliferation of LSCs remains elusive. In the present study, we identified a unique subpopulation of Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) cells coexpressing markers of endothelial cells (including VE-cadherin, PECAM-1, and Flk-1) and committed B-lineage progenitors. After long-term coculture with bone marrow stromal cells, tumor cells formed hematopoietic colonies and cords, expressed early stem- cell markers, and showed endothelial sprouting. Gene expression profiles of LSCs were altered in the presence of stromal cell contact. Stromal cell contact promoted leukemic cell VE-cadherin expression, stabilized β-catenin, and up-regulated Bcr-abl fusion gene expression. Our study indicates that these specific tumor cells are uniquely positioned to respond to microenvironment-derived self-renewing and proliferative cues. Ph+/VE-cadherin+ tumor subpopulation circumvents the requirement of exogenous Wnt signaling for self-renewal through stromal cell support of leukemic cell VE-cadherin expression and up-regulated Bcr-abl tyrosine kinase activity. These data suggest that strategies targeting signals in the marrow microenvironment that amplify the Bcr-abl/VE-cadherin/β-catenin axis may have utility in sensitizing drug-resistant leukemic stem cells.
Introduction
The bone marrow microenvironment plays an important role in maintaining the quiescence and plasticity of hematopoietic stem cells (HSCs). The stem-cell properties are controlled by ligand-receptor signaling and cell-cell adhesion molecules within microenvironmental niches. These anatomical sites predominantly consist of spindle-shaped, N-cadherin –positive endosteal osteoblasts at the bone surface and VE-cadherin–positive sinusoidal endothelium at the thin-walled blood vessels (sinusoids).1,2 The molecular mechanisms underlying interactions between stem cells and their niche are better understood in the context of Drosophila germ stem cells and mouse bone marrow than in humans. In Drosophila ovary, the molecular hinge that anchors germ stem cells to cap cells (niche stromal cells) is E-cadherin and β-catenin.3 In the mouse model of the HSC microenvironment, N-cadherin is expressed in both quiescent HSCs and osteoblasts, and an increase in the number of N-cadherin+ osteoblasts is correlated with an increase in N-cadherin+ HSCs.1,4
Although the effect of bone marrow niches on normal hematopoietic stem and progenitor cells has been extensively investigated, less is known about how the same microenvironment influences leukemic stem cells (LSCs). Although LSCs show a symbiotic relationship with the specialized bone marrow microenvironment,5 the role of bone marrow fibroblastic stromal cells in supporting self-renewal of acute lymphoblastic leukemia (ALL) LSCs remains largely unclear. Recent studies indicated that the canonical Wnt/β-catenin signaling pathway plays a pivotal role in B-lineage hematopoietic stem- and progenitor-cell development.6 In addition to the destruction and transcription complexes involved in the Wnt signaling pathway, β-catenin also exists in the cadherin-catenin-actin cell-cell adhesion complex.7 VE-cadherin (vascular-endothelial cadherin) is one of the classic Ca2+-dependent, homophilic adhesion molecules primarily expressed in endothelial cell adherens junctions. The intracellular domain of VE-cadherin physically interacts with p120 catenin, β-catenin, α-catenin, and the actin cytoskeleton. Tyrosine phosphorylation of the C-terminus of β-catenin at Tyr142, or the VE-cadherin intracellular domain at Tyr 658/731 by Src family kinases, alters the binding affinity of β-catenin to VE-cadherin.8,9
Interplay between β-catenin and cadherin family proteins may modulate the stem-cell properties of normal HSCs. Whether this interaction influences LSC maintenance and progression and how this interaction contributes to sustained leukemic cell self-renewal warrant further investigation. We present data showing that a putative LSC subset of ALL uses the Bcr-abl/VE-cadherin/β-catenin axis to bypass the requirement of externally stimulated Wnt/β-catenin. Although dependence on microenvironment cues for self-renewal is lost, the ability of leukemic cells to respond to stromal signals is maintained. Stromal cells regulate self-renewal and proliferation of this Philadelphia-chromosome positive (Ph+)/VE-cadherin+ LSC-like subpopulation by up-regulation of VE-cadherin and Bcr-abl expression.
Materials and methods
Cells and reagents
Ph+ ALL cell line Sup-B15 (p185 Bcr-abl+) was obtained from ATCC (Manassas, VA). Nalm27 (p210 Bcr-abl+), Nalm20, and Nalm29 ALL cell lines were kindly provided by the Fujisaki Cancer Center, Okayama, Japan. Control cell lines JM1, RS4;11, REH, Jurkat, HL60, and K562 were also from ATCC. Ph+ OP-1 was a gift from Dr Dario Campana (St Jude Children's Research Hospital, Memphis, TN). Maintenance of human bone marrow–derived stromal cell line PatX and murine stromal cell line S-10 (provided by Dr Kenneth Dorshkind of the University of California) has been previously described in detail.10 To establish long-term coculture of stromal and leukemic cells, Sup-B15 and Nalm27 cells were seeded onto 70% confluent stromal cells and maintained by subculture of a portion of Sup-B15 or Nalm27 cells onto new stromal cells weekly for more than 12 months. Bcr-abl kinase inhibitors Imatinib Mesylate (IM) and AG957 were obtained from Novartis (Basel, Switzerland) and Sigma (St Louis, MO), respectively. Src kinase inhibitor PP2 and proteosome inhibitor MG132 were purchased from Calbiochem (San Diego, CA). All recombinant cytokines and growth factors were purchased from R&D Systems (Minneapolis, MN).
Plasmid constructs, lentiviral vectors, and retroviral vectors
Human VE-cadherin (referred to as CDH5/wild type, wt), VE-cadherin lacking the C-terminal 222-nt β-catenin binding domain (referred to as Δbcat), and VE-cadherin lacking the C-terminal 449-nt cytoplasmic domain (referred to as Δcyto) coding DNA sequences (CDSs) were amplified with high-fidelity DNA polymerase pfx (Invitrogen, Carlsbad, CA) from a human umbilical cord vein endothelial cell cDNA library and subsequently ligated into the pENTR-D/TOPO entry vector using the TOPO cloning strategy (Invitrogen). The 2 truncated derivates and the wt VE-cadherin pENTR entry plasmid constructs were recombined with the pLenti6.2-Dest/V5 lentiviral vector by LR recombination using the Gateway cloning technology (Invitrogen). The pLenti6.2-Dest/V5 empty vector control (referred to as vect) was generated by recombination of the destination lentiviral vector with a circularized empty pENTR-D/TOPO plasmid to replace the attR1-CmR-ccdB-attR2 toxic protein from the pLenti6.2-Dest/V5 vector.
The murine stem-cell retroviral (MSCV) vectors pMigR1–210 and pMigR1–185 carrying the Bcr-abl (p210 and p185) full-length fusion gene cDNAs were kindly provided by Dr Ann Marie Pendergast of Duke University. Both plasmid constructs are bicistronic with an internal ribosomal entry site (IRES) upstream of the enhanced green fluorescent protein (eGFP) coding sequence. To generate the matched empty vector control pMSCV-IRES-eGFP (pMiG), the 7.2-kb (kilobase) EcoRI fragment coding for p210 Bcr-abl fusion protein was released, and the vector was religated with T4 DNA ligase.
To construct the β-catenin/Tcf reporter gene system, the synthetic sense and antisense strands of the Tcf-binding sites 1 (TBS-1) and Tcf-binding sites 2 (TBS-2) were denatured at 94°C and annealed at room temperature.11,12 The ds-TBS-1/2 was 3′ adenine-tailed with Taq DNA polymerase at 72°C for 15 minutes followed by in vitro phosphorylation of the 5′-OH ends with T4 polynucleotide kinase. The resultant double-stranded ds-TBS-1/2 was inserted into pGEM/Tesay vector (Promega, Madison, WI) by T/A cloning. The TBS-1/2 inserts flanked by XhoI and HindIII sites were excised from the T/A vector, and the released TBS-1/2 oligonucleotides were ligated into the XhoI-HindIII site of the multiple cloning site in phRL-null Renilla luciferase vector (Promega). To construct the Tcf optimal promoter (TOP)/minimal thymidine kinase (mTK) chimeric promoter upstream of the Renilla luciferase coding sequence, a 531-bp (base pair) BglII-PvuII cohesive-blunt fragment of the thymidine kinase promoter region was deleted from the phRL-TK vector (Promega) and replaced with the BglII-SmaI cohesive-blunt fragment containing the TBS-1/2 fragments released from the phRL-TBS-1/2.
To construct the Bcr-abl promoter reporter, the pMigR1–210 was first digested with BamHI. The resulting BamHI 5′-overhangs were fill-in blunted with Klenow. A 640-bp EcoRI-BamHI fragment (pp210S) and an approximately 1.5-kb BamHI-EcoRI fragment (pp210L), spanning the putative Bcr-abl promoter region and part of the first exon,13 was further released from the linearized pMigR1–210 and inserted directionally (for pp210S) and in reverse direction (for pp210L) into the EcoRI-SmaI site of the phRL-null Renilla luciferase vector.
All the synthetic oligonucleotides for long and accurate–polymerase chain reaction amplification of relevant gene CDSs, or for construction of TOP-mTK chimeric promoters, are indicated in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). At the 5′-end of each forward primer, a CACC 5′-overhang was added to the primer to ensure directional ligation into pENTR-D/TOPO vector and to constitute the Kozak consensus sequence (CACCATGC/G) for efficient translational initiation. At the 3′-end of each reverse primer, the TGA/TAG stop codons were site-directionally mutated into AGA to allow downstream V5 epitope expression. For the Tcf binding sites 1 and 2, the XhoI and HindIII restriction sites are underlined, respectively.
Production of lentiviral and retroviral stocks
To generate replication-incompetent lentiviral particles carrying the CDH5-wt, Δbcat, Δcyto, and vect, the pLenti6.2-Dest/V5 vectors containing the gene CDSs described were cotransfected with pLP1 (gag/pol), pLP2 (rev), and pLP3 (VSV-G, vesicular stomatitis virus-G pseudotype) plasmids (Invitrogen) into 293FT packaging cells (Invitrogen). To generate the replication-incompetent murine stem cell retroviruses expressing the p210/p185 Bcr-abl fusion protein, the pMigR1–210, pMigR1–185, or pMiG empty vector constructs were transfected into the RetroPack PT67 packing cells (BD-Clontech, San Jose, CA), in which the gag/pol, rev, and the pseudotyped VSV-G envelope coding sequences have been stably integrated into the PT67 genome. For detailed protocols of collection, titration, and cell infection, see the data supplement.
Dual-luciferase reporter (DLR) assay
To evaluate transactivation of the β-catenin/Tcf signaling and the Bcr-abl transcription activity initiated by long-term stromal cell coculture or enforced expression of VE-cadherin in Sup-B15 cells, cells were transiently cotransfected in triplicate with pGL4.13-CMV/Luc2 (Promega) firefly luciferase expression vector (as an internal transfection efficiency control) and the Renilla luciferase TOP1/2-mTK or Bcr-abl pp210S/pp210L promoter reporter constructs. For collection, cell lysis, and bioluminescence assay, see the data supplement.
Knockdown of VE-cadherin expression by RNAi
SmartPool short interfering RNAs (siRNAs) corresponding to coding sequences of VE-cadherin was obtained from Dharmacon (Lafayette, CO). Transient siRNA transfection of Sup-B15 cells was completed as previously described with the use of scrambled siRNA sequence controls.10
Endothelial differentiation
Evaluation of endothelial cell differentiation (ie, sprouting) of Sup-B15 and Nalm27 cells was completed as recommended by the manufacturer (Stem Cell Technologies, Toronto, Canada). Briefly, LTCC Ds-Red–labeled Sup-B15 or eGFP-labeled Nalm27 cells were collected and incubated in the EndoCult liquid medium supplemented with EndoCult supplements on cover slips. Forty-eight hours after induction of differentiation, cells were fixed in formaldehyde and analyzed by confocal microscopy.
Microarray analysis
Wnt, Notch, HSC, and cell surface marker pathway-focused microarray analyses of mRNA transcripts in Sup-B15 LTMCs/LTCCs were performed according to the manufacturer's instructions (SuperArray, Frederick, MD). For a brief protocol, see Document S1.
In vitro poly-ubiquitination of β-catenin
In vitro poly-ubiquitination was performed as described previously.14 Briefly, β-catenin was immunoprecipitated either from 293FT cells expressing eGFP-210 or eGFP alone, or from Ph+ Sup-B15 or Ph− REH leukemic cells. The ubiquitin-protein conjugation reaction was performed at 37°C in a waterbath for 3.5 hours in a volume of 20 μL containing 50 mM HEPES, pH 7.5, 2 mM DTT, 5 mM MgCl2, 5 mM ATP, Fraction A (including human E1 and E2 enzymes), Fraction B (containing human E3 enzyme), 10 μg recombinant ubiquitin (BostonBiochem, Boston, MA) and immunoprecipitated β-catenin in the presence of 2 μM ubiquitin C-terminal hydrolase inhibitor ubiquitin aldehyde. The reaction was terminated by the addition of 10 μL 3× SDS sample buffer and boiled at 100°C for 5 minutes.
Confocal microscopy, immunoprecipitation, immunoblotting, and flow cytometry
All confocal images were acquired on a Zeiss LSMS10 confocal system attached to an Axiovert 100M microscope. This system has 3 lasers (488, 543, and 633) and 3 PMTs to collect multiple fluorescent probes. The images were acquired and processed using the Zeiss LSMS10 software version 3.21 (Carl Zeiss, Thornwood, NY). All the antigen-antibody–based experiments were completed as described previously.10,15 Antibodies are described in detail in Document S1. All human-derived stromal cells were established from de-identified samples in compliance with the West Virginia University Institutional Review Board guidelines.
Results
Identification and characterization of a leukemic stem cell–like population that expresses both endothelial and hematopoietic progenitor cell surface markers
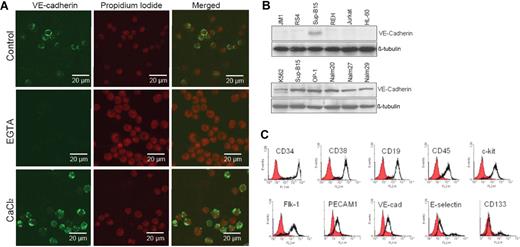
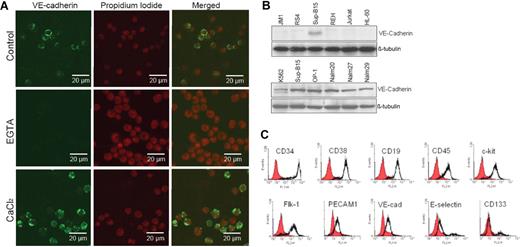
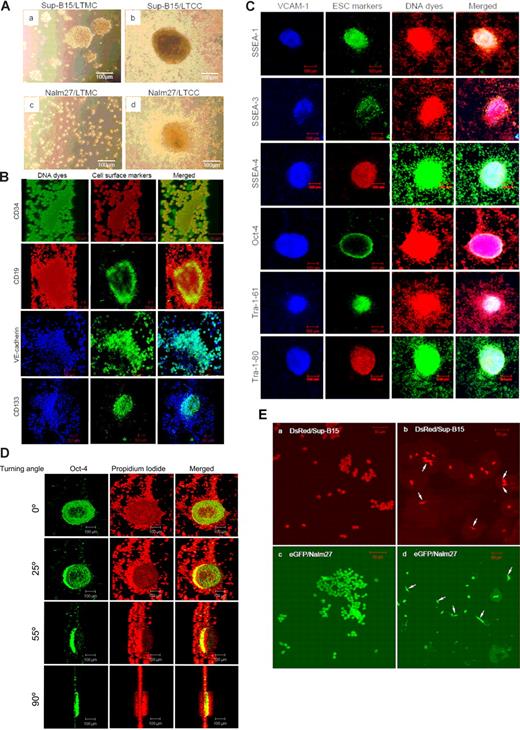
Both p185 (Sup-B15) and p210 (Nalm27) Bcr-abl–positive ALL cells exhibit either as floating aggregates or adherent patches, distinct from Ph− leukemic cells during in vitro culture. Screening a panel of adhesion molecules, including VCAM-1, PECAM-1, ICAM-1, and the cadherin family of proteins (data not shown), in Ph+ ALL cells revealed that Bcr-abl–positive cells uniquely express VE-cadherin. Leukemic cell VE-cadherin was calcium sensitive and was predominantly localized on the cell surface (Figure 1A). Pretreatment of cells with the calcium chelator EGTA diminished the VE-cadherin fluorescent signal on leukemic cells, whereas addition of Ca2+ enhanced the calcium-mediated fluorescent signals (Figure 1A). Expression of VE-cadherin is absent on Ph− ALL cells, whereas all the Ph+ ALL cell lines tested, including K562 Ph+ chronic myelogenous leukemia (CML) (blast crisis), express VE-cadherin (Figure 1B). Reverse transcription–polymerase chain reaction confirmed VE-cadherin expression in Ph+ leukemic cells, but not in Ph− leukemic cells, at the mRNA level (data not shown). Immunophenotypic characterization of the Ph+/VE-cadherin+ subset of ALL cells indicated that hematopoietic stem/progenitor cell surface markers CD34, CD38, and c-kit; mature B-cell lymphoid markers CD19 and CD45; and the endothelial antigens Flk-1, PECAM-1, and VE-cadherin are coexpressed on Sup-B15 cell surface (Figure 1C). Approximately 12% of the Sup-B15 cells also expressed the early endothelial marker CD133 (Figure 1C). Thus, Ph+/VE-cadherin+ identifies a unique subpopulation of ALL cells distinctive from Ph− ALL cell population.
Identification and characterization of a leukemic stem cell–like population that expresses both endothelial and hematopoietic progenitor cell surface markers. (A) Confocal laser scanning microphotographs (LSMs) of Ph+ ALL Sup-B15 cells stained with anti–VE-cadherin in the presence of 10 mM EGTA and 5 mM CaCl2. Cell nuclei were counterstained with propidium iodide (PI) and 1024 × 1024 12-bit confocal images were collected using 40×/0.75 Plan -Neofluar objective. (B) Cell lysates prepared from Ph− acute leukemia cell lines JM1, RS4;11, REH, Jurkat, HL60, and Ph+ acute leukemia cell lines K562, Sup-B15, OP-1, Nalm20, Nalm27, and Nalm29 were Western blotted with anti–VE-cadherin. The same membranes were stripped and reprobed with anti–β-tubulin as a loading control. (C) Immunophenotyping of Ph+/VE-cadherin+ Sup-B15 cells by flow cytometry. Cells were surface stained to evaluate hematopoietic stem/progenitor cell and classic endothelial markers.
Identification and characterization of a leukemic stem cell–like population that expresses both endothelial and hematopoietic progenitor cell surface markers. (A) Confocal laser scanning microphotographs (LSMs) of Ph+ ALL Sup-B15 cells stained with anti–VE-cadherin in the presence of 10 mM EGTA and 5 mM CaCl2. Cell nuclei were counterstained with propidium iodide (PI) and 1024 × 1024 12-bit confocal images were collected using 40×/0.75 Plan -Neofluar objective. (B) Cell lysates prepared from Ph− acute leukemia cell lines JM1, RS4;11, REH, Jurkat, HL60, and Ph+ acute leukemia cell lines K562, Sup-B15, OP-1, Nalm20, Nalm27, and Nalm29 were Western blotted with anti–VE-cadherin. The same membranes were stripped and reprobed with anti–β-tubulin as a loading control. (C) Immunophenotyping of Ph+/VE-cadherin+ Sup-B15 cells by flow cytometry. Cells were surface stained to evaluate hematopoietic stem/progenitor cell and classic endothelial markers.
Ph+/VE-cadherin+ leukemic cells form hematopoietic colonies/cords on bone marrow niche stromal cells and differentiate into endothelial cells in vitro
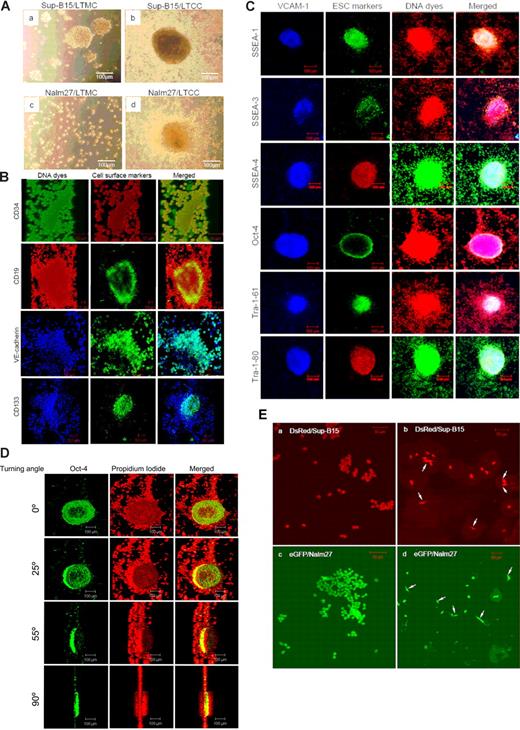
Long-term coculture (LTCC) of Sup-B15 or Nalm27 leukemic cells on either S10 or PatX stromal cells supported the formation of hematopoietic colonies (“hemospheres”; Figure 2A), resembling neurospheres generated by neural stem cells or mamospheres formed by breast cancer stem cells.16,17 Hemospheres formed on stromal cells structurally resembled both type I (compact colony; Figure 2A upper right) and type II (colony with a dense center surrounded by migrating cells; Figure 2A lower right) but not type III (diffuse colonies of mobile differentiating cells) as characterized previously.18,19 However, unlike cancer stem cell colonies derived from solid tumors, formation of hemospheres from Ph+/VE-cadherin+ leukemic cells are stromal cell dependent, with hemospheres only initiated in physical association with a monolayer of VCAM-1–positive stromal cells. Of all the ALL cell lines tested, only the Ph+ subset was capable of hemosphere formation, whereas Ph− ALL cell lines did not form hemospheres during LTCC for up to 6 months (data not shown). Moreover, hemospheres are resistant to EDTA-free trypsin digestion during regular subculture, whereas leukemic cell aggregates in long-term medium culture (LTMC) can be disrupted by mechanic vibration.
Ph+/VE-cadherin+ leukemic cells form hematopoietic colonies/cords on bone marrow niche stromal cells and differentiate into endothelial cells in vitro. (A) Phase-contrast micrographs of Ph+ ALL Sup-B15 (top) and Nalm27 (bottom) cells cultured in medium alone (long-term medium culture, LTMC) or long-term cocultured (LTCC) on murine stromal cell line S10 (top and bottom right). Images were collected by using a Leica DMIL microscope and 10×/0.22 objective (Houston, TX). (B) Confocal LSM microphotographs of hematopoietic cords formed on stromal cells. Cells were surface stained with anti-CD34, CD19, VE-cadherin, and CD133, respectively. Nucleic DNA dyes include Sytox (green), PI (red), and TO-PRO-3 (blue). Z stacks were 3-D reconstructed with LSM510 software (version 3.2; Zeiss, Jena, Germany). The turning axis is the y-axis, and turning angle for the displayed static pictures is 45° and confocal images were obtained using 20×/0.75 Fluar objective (rows 1, 3) and 10×/0.50 Fluar objective (rows 2, 4). (C) Confocal micrographs of hematopoietic colonies (“hemospheres”) stained with a panel of early stem cell (ESC) markers. Stromal monolayer cells underneath hemospheres were counterstained with anti–VCAM-1. Cell nuclei were counterstained with DNA dyes PI (red fluorescence) or SYTOX Green (green fluorescence) to be compatible with the fluorochrome tag of the secondary antibodies of anti-ESC antibody. Merged images of 3-channel colors are shown in the final column and images were acquired using a 10×/0.5 Fluar objective. (D) Three-dimensional reconstruction of z-stacks showing the distribution of transcriptional factor Oct-4 within a hematopoietic cord. Cell nuclei were counterstained with PI (red), and localization of Oct-4 was labeled with green fluorescence. The turning axis is the y-axis. The tip of the hematopoietic cord was pressed flat by the cover slip and images were acquired using a 10×/0.5 Fluar objective. (E) DsRed red fluorescent protein-–labeled Sup-B15 cells (top left) or eGFP-labeled Nalm27 cells (bottom left) were cultured in standard medium or in EndoCult endothelial-defined medium (top and bottom right). White arrows denote individual endothelial sprouting observed for individual Ph+/VE-cadherin+ ALL cells.
Ph+/VE-cadherin+ leukemic cells form hematopoietic colonies/cords on bone marrow niche stromal cells and differentiate into endothelial cells in vitro. (A) Phase-contrast micrographs of Ph+ ALL Sup-B15 (top) and Nalm27 (bottom) cells cultured in medium alone (long-term medium culture, LTMC) or long-term cocultured (LTCC) on murine stromal cell line S10 (top and bottom right). Images were collected by using a Leica DMIL microscope and 10×/0.22 objective (Houston, TX). (B) Confocal LSM microphotographs of hematopoietic cords formed on stromal cells. Cells were surface stained with anti-CD34, CD19, VE-cadherin, and CD133, respectively. Nucleic DNA dyes include Sytox (green), PI (red), and TO-PRO-3 (blue). Z stacks were 3-D reconstructed with LSM510 software (version 3.2; Zeiss, Jena, Germany). The turning axis is the y-axis, and turning angle for the displayed static pictures is 45° and confocal images were obtained using 20×/0.75 Fluar objective (rows 1, 3) and 10×/0.50 Fluar objective (rows 2, 4). (C) Confocal micrographs of hematopoietic colonies (“hemospheres”) stained with a panel of early stem cell (ESC) markers. Stromal monolayer cells underneath hemospheres were counterstained with anti–VCAM-1. Cell nuclei were counterstained with DNA dyes PI (red fluorescence) or SYTOX Green (green fluorescence) to be compatible with the fluorochrome tag of the secondary antibodies of anti-ESC antibody. Merged images of 3-channel colors are shown in the final column and images were acquired using a 10×/0.5 Fluar objective. (D) Three-dimensional reconstruction of z-stacks showing the distribution of transcriptional factor Oct-4 within a hematopoietic cord. Cell nuclei were counterstained with PI (red), and localization of Oct-4 was labeled with green fluorescence. The turning axis is the y-axis. The tip of the hematopoietic cord was pressed flat by the cover slip and images were acquired using a 10×/0.5 Fluar objective. (E) DsRed red fluorescent protein-–labeled Sup-B15 cells (top left) or eGFP-labeled Nalm27 cells (bottom left) were cultured in standard medium or in EndoCult endothelial-defined medium (top and bottom right). White arrows denote individual endothelial sprouting observed for individual Ph+/VE-cadherin+ ALL cells.
Formation of flamelike, finger-shaped hematopoietic cords from Ph+/VE-cadherin+ LTCC on both human and murine stromal cells follows formation and growth of hemospheres (Figure 2B; Video clips S1–S6). Hematopoietic cords were characterized by a bamboo shootlike structure composed of distinct cellular constituents localized to the peripheral or central regions (Figure 2B). In a typical hematopoietic cord, the inner column is composed of CD133+VE-cadherin+ cells with the periphery of the cord constituted by more differentiated CD133-VE-cadherin+ leukemic cells (Figure 2B; Video clips S3–S4). To further examine the stem cell potential of the leukemic cells that comprised the hemospheres and hematopoietic cords, we stained the hemosphere and hematopoietic cord cells with early stem cell (ESC) markers (Figure 2C,D). Confocal microscopy revealed distinct expression and distribution patterns of ESC markers. Oct-4 was uniquely expressed in the peripheral region of the hemospheres (Figure 2D), whereas SSEA-1–, SSEA-3–, SSEA-4–, Tra-1–61–, and Tra-1–80–positive cells were limited to the central region (Figure 2C). Migratory leukemic cells surrounding the hemospheres were negative for ESC marker staining (Figure 2C,D). When LTCC Ph+/VE-cadherin+ cells were incubated in EndoCult, we observed endothelial sprouting of Ph+VE-cadherin+ Sup-B15 or Nalm 27 cells (Figure 2E). Many Ph+VE-cadherin+ LTCC cells differentiated from round, floating cells to spindle-shaped, adherent cells within 2 days (Figure 2E). Thus, deprivation of stromal cell support in the presence of endothelial-trophic growth factors leads to a rapid endothelial switch of Ph+/VE-cadherin+ cells after long-term stromal cell contact.
LTCC with bone marrow stromal cells promotes self-renewal activity of Ph+/VE-cadherin+ leukemic cells independent of the Wnt/β-catenin signaling pathway
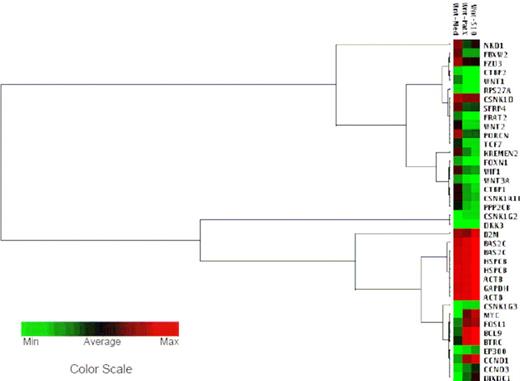
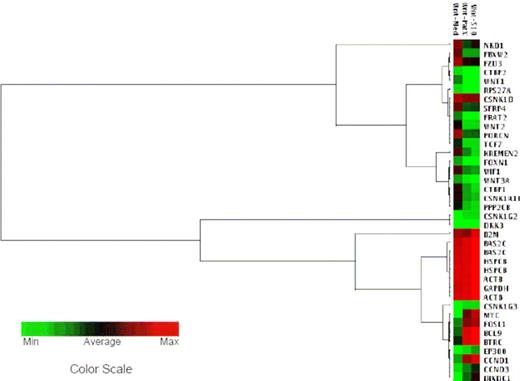
To investigate the signaling pathway(s) potentially involved in the stem cell–like phenotypes of Ph+/VE-cadherin+ ALL cells after LTCC with stromal cells, pathway-focused microarrays were completed to compare the gene expression profiles in LTMC and LTCC Sup-B15. Sup-B15 genes involved in the Wnt/β-catenin signaling pathway respond to S10 or PatX stromal cell signals differentially (Figure 3; Table S2). Tcf/β-catenin target genes, including cyclin D1 (CCND1), cyclin D3 (CCND3), and c-Myc (MYC), were increased by 2- to 2.5-fold, 2- to 2.5-fold, and 3- to 3.5-fold, respectively, after LTCC. The β-catenin transcriptional coactivator Bcl-9 (BCL9) was up-regulated 1.5- to 2-fold, whereas its transcriptional corepressor Tcf (TCF7) and the Wnt inhibitory factor 1 (WIF1) were down-regulated 2- and 1.5-fold, respectively. However, expression of the intracellular signaling mediator β-catenin (CTNNB1) and β-catenin binding protein 1 (CTNNB1P1) remained unchanged. Further, the leukemic cell autocrine Wnts, including Wnt1, Wnt2, and Wnt3A, were down-regulated by 2- to 2.3-fold in LTCC Sup-B15 compared with those of LTMC Sup-B15, which were coincident with down-regulation of the Wnt coreceptor Frizzled (FZD3; 1.5-fold) and the secretory Wnt precursor-processing protein (PORCN; 2-fold). Thus, Wnt signaling cascades downstream of ligand/receptor occupancy were aberrantly intercepted and reinforced, whereas the extracellular component of the signaling pathway was attenuated or disabled in Ph+/VE-cadherin+ ALL cells during LTCC.
LTCC with bone marrow stromal cells promotes self-renewal activity of Ph+/VE-cadherin+ leukemic cells independent of the Wnt/β-catenin signaling pathway. Clustering analysis of gene expression profiles in Sup-B15 cells after LTCC with S10 or PatX stromal cells. The hierarchical clusters were created with GEASuite software based on the similarity of gene expression. The green color at the farthest left end of the color scale corresponds to the minimal value; the red color at the farthest right end of the color scale corresponds to the maximum value, black corresponds to the average value of expression. BAS2C, HSPCB, ACTB, and GAPDH served as housekeeping gene controls. Analysis of the representative Wnt pathway is shown.
LTCC with bone marrow stromal cells promotes self-renewal activity of Ph+/VE-cadherin+ leukemic cells independent of the Wnt/β-catenin signaling pathway. Clustering analysis of gene expression profiles in Sup-B15 cells after LTCC with S10 or PatX stromal cells. The hierarchical clusters were created with GEASuite software based on the similarity of gene expression. The green color at the farthest left end of the color scale corresponds to the minimal value; the red color at the farthest right end of the color scale corresponds to the maximum value, black corresponds to the average value of expression. BAS2C, HSPCB, ACTB, and GAPDH served as housekeeping gene controls. Analysis of the representative Wnt pathway is shown.
Stromal cells up-regulate VE-cadherin expression and stabilize β-catenin in Ph+/VE-cadherin+ cells
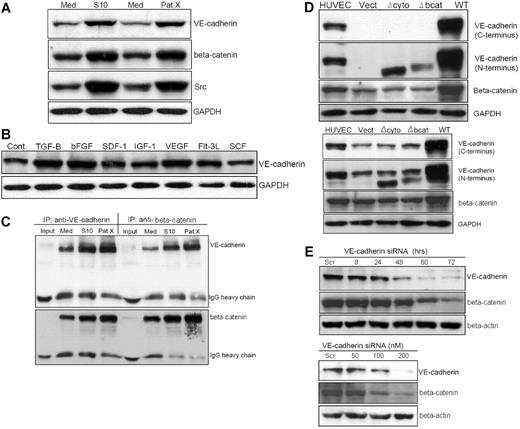
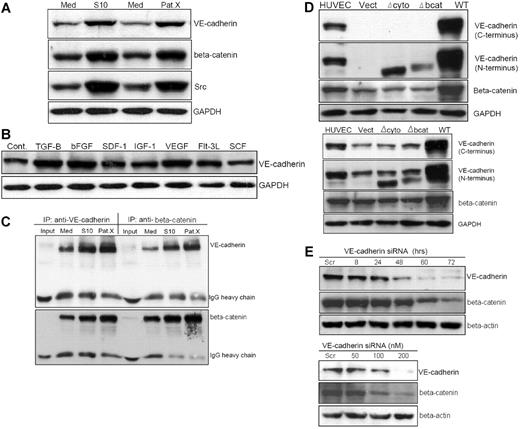
The convergence of β-catenin in cadherin-catenin–mediated cell-cell adhesion and morphogenesis, and as an intracellular mediator of the canonical Wnt signaling pathway, prompted us to hypothesize that VE-cadherin expression may contribute to self-renewal and proliferation of Ph+/VE-cadherin+ cells. After LTCC of Sup-B15 with both murine and human marrow stromal cells, VE-cadherin expression was up-regulated, coincident with elevated Src expression and β-catenin accumulation (Figure 4A). Screening a panel of stromal cell–soluble growth factors and cytokines showed that TGF-β1, bFGF, and VEGF stimulated VE-cadherin expression on Sup-B15 cells by 2.0-, 1.50-, and 1.8-fold, respectively (Figure 4B). To determine whether up-regulated VE-cadherin contributes to stabilizing β-catenin in Ph+/VE-cadherin+ ALL cells, reciprocal coimmunoprecipitation revealed that the amount of β-catenin complexed with VE-cadherin in tumor cells derived from either S10 or PatX LTCC were 2- to 3.5-fold higher than those from LTMC alone (Figure 4C). Because microarray data indicated that β-catenin expression is not influenced by stromal cell contact (Figure 3), we hypothesized that up-regulated VE-cadherin plays a major role in sequestering and stabilizing β-catenin in Ph+/VE-cadherin+ cells. To investigate this possibility, lentiviral transduction of Sup-B15 and 293FT cells with vectors carrying the wild-type and 2 mutant forms of VE-cadherin, Δbcat, and Δcyto, were established (Figure 4D). Expression of the wt VE-cadherin, but not VE-cadherin lacking either the cytoplasmic or β-catenin binding domains, led to approximately 4-fold more β-catenin accumulation in both 293FT (Figure 4D top) and Sup-B15 (Figure 4D bottom) cells. Thus, stabilization of β-catenin in the presence of VE-cadherin may be a common molecular event in nonendothelial cells. To further investigate the relation between VE-cadherin and β-catenin in Ph+/VE-cadherin+ leukemic cells, VE-cadherin siRNA or scrambled control dsRNA was transiently transfected into Sup-B15 cells. Knockdown of VE-cadherin expression in Sup-B15 cells resulted in diminished β-catenin in a time-dependent (Figure 4E top) and concentration-dependent (Figure 4E bottom) manner. Therefore, bone marrow niche stromal cells may modulate Ph+/VE-cadherin+ leukemic cell self-renewal activity, at least in part, by influencing VE-cadherin expression and subsequent β-catenin stabilization.
Stromal cells up-regulate VE-cadherin expression and stabilize β-catenin in Ph+/VE-cadherin+ leukemic cells. (A) Western blot analysis of Sup-B15 cells in LTMC (Med) or in LTCC with either S10 (S10) or PatX (PatX) stromal cells. The same membrane was probed with anti–VE-cadherin, anti–β-catenin, and anti-Src, respectively. GAPDH was used as the lane loading control. (B) Western blot of cell lysates isolated from Sup-B15 cells exposed to recombinant human cytokines, chemokines, or growth factors compared with untreated control cells (Cont). (C) Reciprocal coimmunoprecipitation of Sup-B15 cell lysates from LTMC (Med) or LTCC (S10 and PatX) with specific antibodies for anti–VE-cadherin or anti–β-catenin. Membranes were reciprocally probed with anti–β-catenin and anti–VE-cadherin, respectively. IgG heavy chains served as the loading control. Input denotes sample with antibody alone, lacking cell lysate. (D) Western blot analysis of cell lysates from 293FT (top) or Sup-B15 cells (bottom) transduced with lentiviruses carrying the wild-type VE-cadherin (WT) or VE-cadherin lacking either the cytoplasmic domain (Δcyto) or β-catenin binding domain (Δbcat) CDSs (coding DNA sequences). Vect denotes the empty lentiviral vector encoding the blasticidin resistance protein without a CDS insert. (E) Western blot analysis of Sup-B15 cells transiently transfected with VE-cadherin siRNA or scrambled (Scr) control siRNA sequence. Time-course (top) and dose-response (bottom) of VE-cadherin siRNA-transfected samples were probed with specific antibodies for anti–VE-cadherin and β-catenin.
Stromal cells up-regulate VE-cadherin expression and stabilize β-catenin in Ph+/VE-cadherin+ leukemic cells. (A) Western blot analysis of Sup-B15 cells in LTMC (Med) or in LTCC with either S10 (S10) or PatX (PatX) stromal cells. The same membrane was probed with anti–VE-cadherin, anti–β-catenin, and anti-Src, respectively. GAPDH was used as the lane loading control. (B) Western blot of cell lysates isolated from Sup-B15 cells exposed to recombinant human cytokines, chemokines, or growth factors compared with untreated control cells (Cont). (C) Reciprocal coimmunoprecipitation of Sup-B15 cell lysates from LTMC (Med) or LTCC (S10 and PatX) with specific antibodies for anti–VE-cadherin or anti–β-catenin. Membranes were reciprocally probed with anti–β-catenin and anti–VE-cadherin, respectively. IgG heavy chains served as the loading control. Input denotes sample with antibody alone, lacking cell lysate. (D) Western blot analysis of cell lysates from 293FT (top) or Sup-B15 cells (bottom) transduced with lentiviruses carrying the wild-type VE-cadherin (WT) or VE-cadherin lacking either the cytoplasmic domain (Δcyto) or β-catenin binding domain (Δbcat) CDSs (coding DNA sequences). Vect denotes the empty lentiviral vector encoding the blasticidin resistance protein without a CDS insert. (E) Western blot analysis of Sup-B15 cells transiently transfected with VE-cadherin siRNA or scrambled (Scr) control siRNA sequence. Time-course (top) and dose-response (bottom) of VE-cadherin siRNA-transfected samples were probed with specific antibodies for anti–VE-cadherin and β-catenin.
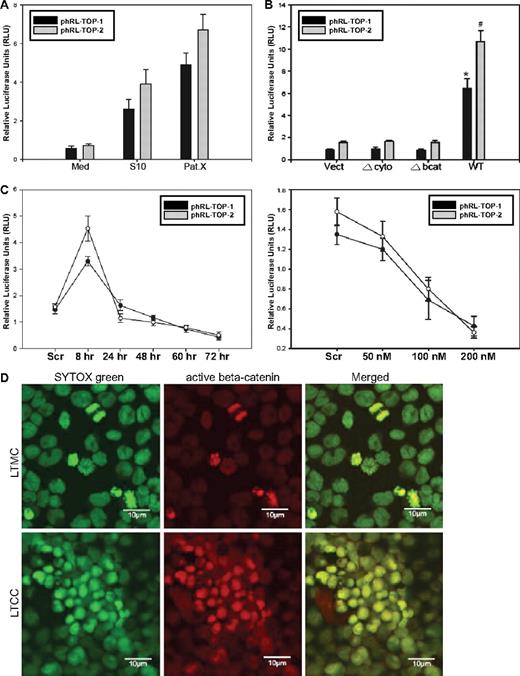
β-Catenin is constitutively activated in leukemic stem-like cells that overexpress VE-cadherin during LTCC
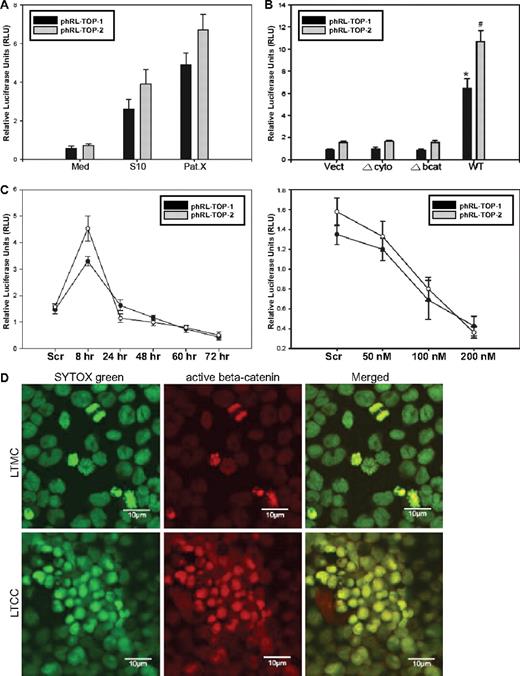
To examine the β-catenin/Tcf transcriptional activity, Sup-B15 LTMC or LTCC cells were transiently cotransfected with phRL-TOP1 or phRL-TOP2 (Renilla luciferase) with an internal control plasmid, pGL4.13-CMV-Luc2 (firefly luciferase). Stromal cell contact resulted in a 5- to 13-fold increase in relative luciferase activity in LTCC (Figure 5A). Moreover, enforced expression of the wt, but not Δbcat and Δcyto, VE-cadherin significantly promoted β-catenin/Tcf transcriptional activity in Sup-B15 cells (Figure 5B). In contrast, depletion of VE-cadherin expression by siRNA resulted in an unexpected, transient elevation of β-catenin activity during the first 8 hours after transfection, followed by a persistent declining in relative luciferase activities in a time-dependent (Figure 5C top) and siRNA concentration-dependent (Figure 5C bottom) manner. One interpretation of this interesting phenomenon is that the initial reduction of the docking protein VE-cadherin releases β-catenin capable of driving luciferase reporter expression because of the release of excessive, free β-catenin that cannot be efficiently targeted for proteosome-mediated degradation. However, long-term reduction of VE-cadherin may diminish its capability to protect β-catenin from targeted ubiquitination and degradation that is typically achieved through physical interaction. Stromal cell signals also altered the intracellular distribution of β-catenin in Ph+/VE-cadherin+ cells. Translocation of β-catenin from the cytoplasm to the nucleus in Sup-B15 cells was sporadically observed in actively dividing cells in LTMC (Figure 5D); however, long-term coculture of Sup-B15 cells consistently promoted nuclear translocation of β-catenin in most of the leukemic cells on hematopoietic cords (Figure 5D).
β-Catenin is constitutively activated in leukemic stem-like cells overexpressing VE-cadherin during LTCC. (A) Dual-luciferase reporter (DLR) assays of Sup-B15 LTMC (Med) or LTCC cells (S10 or PatX) transiently cotransfected with phRL-TOP1 or phRL-TOP2 Renilla luciferase reporters with an internal control pGL4.13-CMV-Luc2 firefly luciferase reporter. Experimental Renilla luciferase activity was normalized to that of control firefly luciferase and is shown as relative luciferase units (RLUs). Data were presented as mean plus or minus SD (n = 3). (B) DLR assays of cell lysates from Sup-B15 cells transduced with wild-type or the 2 truncated forms of VE-cadherin. Data were presented as mean plus or minus SD (n = 3). The * denotes significant differences compared with Vect controls (P < .01) based on the Student t test. (C) Sup-B15 cells transfected with 100 nM VE-cadherin siRNA for 8 to 72 hours (top) or at a concentration of 50 to 200 nM for 24 hours (bottom). Data are shown as the mean plus or minus SD (n = 3). (D) Confocal micrographs of LTMC or LTCC Sup-B15 cells stained with anti-active β-catenin antibody and were acquired using a 63×/1.2 water C-Apochromat objective. Cell nuclei were counterstained with SYTOX Green. The photograph of LTMC was made from cytospin preparation, and the photograph of LTCC Sup-B15 cells was taken from a portion of 1 hematopoietic cord.
β-Catenin is constitutively activated in leukemic stem-like cells overexpressing VE-cadherin during LTCC. (A) Dual-luciferase reporter (DLR) assays of Sup-B15 LTMC (Med) or LTCC cells (S10 or PatX) transiently cotransfected with phRL-TOP1 or phRL-TOP2 Renilla luciferase reporters with an internal control pGL4.13-CMV-Luc2 firefly luciferase reporter. Experimental Renilla luciferase activity was normalized to that of control firefly luciferase and is shown as relative luciferase units (RLUs). Data were presented as mean plus or minus SD (n = 3). (B) DLR assays of cell lysates from Sup-B15 cells transduced with wild-type or the 2 truncated forms of VE-cadherin. Data were presented as mean plus or minus SD (n = 3). The * denotes significant differences compared with Vect controls (P < .01) based on the Student t test. (C) Sup-B15 cells transfected with 100 nM VE-cadherin siRNA for 8 to 72 hours (top) or at a concentration of 50 to 200 nM for 24 hours (bottom). Data are shown as the mean plus or minus SD (n = 3). (D) Confocal micrographs of LTMC or LTCC Sup-B15 cells stained with anti-active β-catenin antibody and were acquired using a 63×/1.2 water C-Apochromat objective. Cell nuclei were counterstained with SYTOX Green. The photograph of LTMC was made from cytospin preparation, and the photograph of LTCC Sup-B15 cells was taken from a portion of 1 hematopoietic cord.
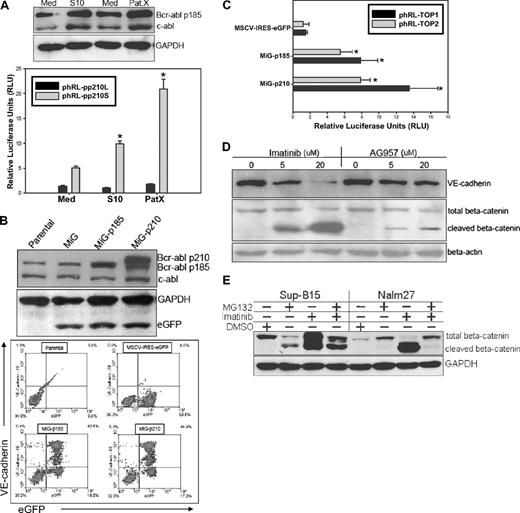
Bcr-abl fusion protein is essential for maintaining the VE-cadherin/β-catenin axis in the leukemic stemlike cells
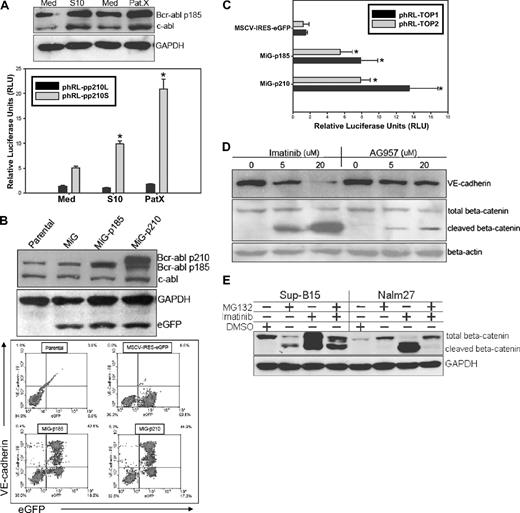
In 293FT cells transduced with various forms of VE-cadherin–expressing lentiviral vectors, forced wild-type VE-cadherin stabilized β-catenin compared with vector control, Δbcat, or Δcyto VE-cadherin (Figure 4D top). However, the β-catenin/Tcf transcriptional activity did not dramatically change after transduction of vect, Δbcat, Δcyto, or even wt VE-cadherin in 293FT cells (data not shown). Therefore, VE-cadherin expression alone is probably necessary, but not sufficient, to maintain enhanced β-catenin activity in VE-cadherin+ leukemic cells. We hypothesized that the Bcr-abl fusion protein, with constitutive tyrosine kinase activity, may synergize with VE-cadherin expression to contribute to the unique phenomenon of leukemic cell self-renewal and proliferation. To test this, we examined the alteration of Bcr-abl protein expression in LTMC and LTCC leukemic cells and observed that stromal cell contact up-regulated both Abl and Bcr-abl protein expression by 1.5- to 2-fold (Figure 6A top). To confirm the observation at the transcriptional level, the Bcr-abl fusion gene promoter region was cloned into the phRL-null luciferase reporter vector. The relative luciferase activity was up-regulated 2- to 4-fold in Sup-B15 LTCC with S10 or PatX as assayed by the pp210S, but not the pp210L, reporter (Figure 6A bottom). To further investigate the role of the Bcr-abl fusion protein, Sup-B15 cells were infected with retroviruses carrying either the p185 or p210 Bcr-abl full-length cDNA. Retroviral transduction of both p185 and p210 Bcr-abl resulted in forced expression of p185 or p210 protein in Sup-B15 cells (Figure 6B top). In Sup-B15 cells overexpressing the p185-eGFP or p210-eGFP proteins, up-regulation of VE-cadherin was also detected as above the parental baseline (Figure 6B bottom). Consistent with up-regulated VE-cadherin expression, β-catenin/Tcf activity was also increased 7- to 14-fold in p185-eGFP– and p210-eGFP–expressing cells compared with eGFP expression alone (Figure 6C). In contrast, inhibition of Bcr-abl activity with the tyrosine kinase inhibitors IM or AG957 down-regulated VE-cadherin expression in a concentration-dependent fashion in Sup-B15 cells, coincident with β-catenin cleavage (Figure 6D). In contrast, treatment of Ph+/VE-cadherin+ leukemic cells with the proteosome inhibitor MG132 diminished imatinib mesylate–induced cleavage of β-catenin in both Sup-B15 and Nalm27 cells (Figure 6E).
Bcr-abl fusion protein is essential for maintaining the VE-cadherin/β-catenin axis in Ph+ ALL. (A top) Western blot analysis of Bcr-abl expression by Sup-B15 in LTMC (Med) or LTCC (S10 or PatX). (Bottom) DLR assays of LTMC (Med) and LTCC (S10 and PatX) Sup-B15 cells transiently cotransfected with the Bcr-abl promoter reporters phRL-pp210L or phRL-pp210S with an internal control pGL4.13-CMV-Luc2 firefly luciferase reporter. Data were shown as mean plus or minus SD (n = 3). The * denotes statistically significant differences compared with values from cells in media alone (P < .01) based on the Student t test. (B) Western blot analysis of Bcr-abl expression by Sup-B15 cells transduced with retroviruses containing p185-eGFP or p210-eGFP forms of Bcr-abl, or the empty vector control (MSCV-IRES-eGFP, MiG) alone (top Western blot). Dual-color flow cytometric analysis of VE-cadherin expression in Sup-B15 cells overexpressing the exogenous Bcr-abl fusion proteins or empty retroviral control vector (bottom flow cytometry). Baseline VE-cadherin was set to zero for comparison of the net increases after retroviral transduction. (C) DLR assay of Sup-B15 cells infected with p185-eGFP, p210-eGFP or MiG vector control retroviruses. Data are shown as mean plus or minus SD (n = 3). The * denotes significant differences compared with MSCV empty vector controls (P < .01) based on the Student t test. (D) Western blot analysis of cell lysates from Sup-B15 cells treated with the Bcr-abl kinase inhibitors Imatinib Mesylate or AG957 at various concentrations. (E) Western blot of Sup-B15 or Nalm27 leukemic cells treated with 20 μM imatinib mesylate, 10 μM proteosome inhibitor MG132, or a combination of both inhibitors for 16 hours.
Bcr-abl fusion protein is essential for maintaining the VE-cadherin/β-catenin axis in Ph+ ALL. (A top) Western blot analysis of Bcr-abl expression by Sup-B15 in LTMC (Med) or LTCC (S10 or PatX). (Bottom) DLR assays of LTMC (Med) and LTCC (S10 and PatX) Sup-B15 cells transiently cotransfected with the Bcr-abl promoter reporters phRL-pp210L or phRL-pp210S with an internal control pGL4.13-CMV-Luc2 firefly luciferase reporter. Data were shown as mean plus or minus SD (n = 3). The * denotes statistically significant differences compared with values from cells in media alone (P < .01) based on the Student t test. (B) Western blot analysis of Bcr-abl expression by Sup-B15 cells transduced with retroviruses containing p185-eGFP or p210-eGFP forms of Bcr-abl, or the empty vector control (MSCV-IRES-eGFP, MiG) alone (top Western blot). Dual-color flow cytometric analysis of VE-cadherin expression in Sup-B15 cells overexpressing the exogenous Bcr-abl fusion proteins or empty retroviral control vector (bottom flow cytometry). Baseline VE-cadherin was set to zero for comparison of the net increases after retroviral transduction. (C) DLR assay of Sup-B15 cells infected with p185-eGFP, p210-eGFP or MiG vector control retroviruses. Data are shown as mean plus or minus SD (n = 3). The * denotes significant differences compared with MSCV empty vector controls (P < .01) based on the Student t test. (D) Western blot analysis of cell lysates from Sup-B15 cells treated with the Bcr-abl kinase inhibitors Imatinib Mesylate or AG957 at various concentrations. (E) Western blot of Sup-B15 or Nalm27 leukemic cells treated with 20 μM imatinib mesylate, 10 μM proteosome inhibitor MG132, or a combination of both inhibitors for 16 hours.
Tyrosine phosphorylation of β-catenin mediated by Bcr-abl kinase diminishes its ubiquitination-proteosome–dependent degradation
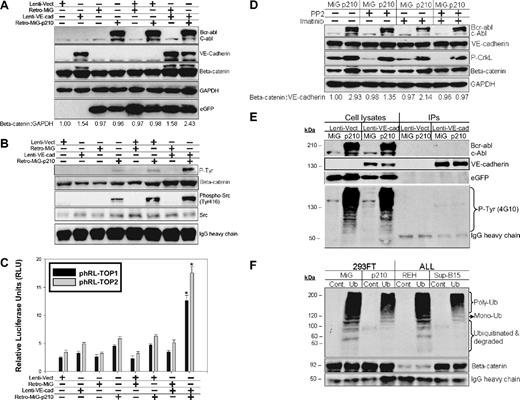
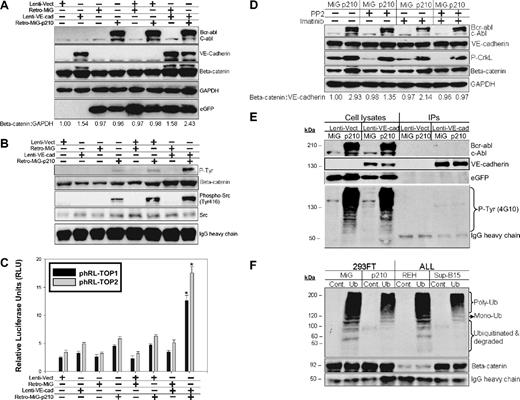
To dissect the signaling pathway by which constitutive Bcr-abl kinase activity maintains higher VE-cadherin/β-catenin in Ph+/VE-cadherin+ ALL cells, Ph−/VE-cadherin− 293FT cells were cotransduced with lentiviruses encoding VE-cadherin and retroviruses encoding the p210 Bcr-abl fusion protein. VE-cadherin/p210 dual transduction resulted in the most pronounced stabilization of β-catenin in 293FT cells compared with VE-cadherin transduction alone (Figure 7A). Immunoprecipitation of β-catenin from the same samples indicated that β-catenin and Src coexisted in the same immunocomplexes and that β-catenin was tyrosine-phosphorylated after transduction with p210 Bcr-abl kinase (Figure 7B). This was consistent with the most pronounced β-catenin transcriptional activity being detected in p210 and VE-cadherin cotransduced cells (Figure 7C). Inhibition of Src activity with PP2, or Bcr-abl with imatinib mesylate, was correlated with modest reduction of β-catenin recognized by a monoclonal antibody specific for its active form. The combinatory inhibition of Src and Bcr-abl kinase activity was associated with a further reduction in β-catenin (Figure 7D). VE-cadherin was immunoprecipitated from the same panel. Although expression of Bcr-abl protein resulted in a number of tyrosine-phosphorylated proteins, VE-cadherin appeared not to be affected by this constitutively active kinase (Figure 7E). To investigate whether tyrosine-phosphorylated β-catenin is more stable or resistant to the ubiquitin-proteosome–mediated protein degradation pathway, β-catenin that was immunoprecipitated from Ph+/− leukemia or 293FT cells expressing eGFP-p210/eGFP was subjected to an in vitro ubiquitination and degradation assay. In the presence of endogenous (Ph+) or exogenously expressed Bcr-abl (eGFP-Bcr-abl), β-catenin had less mono-, poly-ubiquitination, and degraded fractions. This effect was shown by comparison of Ph+Sup-B15 or 293FT/eGFP-p210 to Ph− REH or 293FT/eGFP cells (Figure 7F).
Tyrosine phosphorylation of β-catenin mediated by Bcr-abl kinase diminishes β-catenin ubiquitination-proteosome–dependent degradation. (A) Western blot analysis of cell lysates from 293FT cells cotransduced with lentiviral vector (Lenti-Vect), lentiviruses carrying WT VE-cadherin (Lenti-VE-cad), retroviral vector (Retro-MiG), or retroviruses encoding p210-eGFP (Retro-MiG-p210). β-Catenin was densitometrically normalized to GAPDH with the control value set at 1.0, and all other values are shown relative to the control. (B) Immunoprecipitation of β-catenin was done using the above-mentioned cell lysates, and Western blots were probed with anti–P-Tyr (Phospho-Tyr102), anti–β-catenin, anti–phospho-Src, and anti-Src antibodies. IgG heavy chain served as the loading control. (C) DLR assays from the above 293FT cells transiently cotransfected with the phRL-TOP1 and phRL-TOP2 reporters and an internal pGL4.13-CMV-Luc2 firefly luciferase reporter. Data are shown as mean plus or minus SD (n = 3). The * denotes significant differences compared with vector controls (P < .01) based on the Student t test. (D) Western blot analysis of β-catenin stabilization from 293FT cells coexpressing VE-cadherin/eGFP-p210 or VE-cadherin/eGFP treated with 10 μM Src kinase inhibitor PP2, 10 μM Bcr-abl inhibitor imatinib mesylate, or both inhibitors for 16 hours. β-Catenin was densitometrically normalized to VE-cadherin with the untreated control value of expressing MiG vector alone set to 1.0. All other ratios are normalized to this control value. (E) VE-cadherin was immunoprecipitated from 293FT cells coexpressing VE-cadherin/eGFP-p210 or VE-cadherin/eGFP. The immunoprecipitates and cell lysates were loaded in parallel and probed with anti–phospho-Tyrosine (4G10) and VE-cadherin, c-Abl and eGFP antibodies. (F) β-Catenin immunoprecipitated either from 293FT expressing eGFP-210 or eGFP alone, or from Ph+ Sup-B15 or Ph− REH leukemic cells was in vitro labeled with recombinant ubiquitin and subjected to in vitro degradation assay as described in “In vitro poly-ubiquitination of β-catenin.”
Tyrosine phosphorylation of β-catenin mediated by Bcr-abl kinase diminishes β-catenin ubiquitination-proteosome–dependent degradation. (A) Western blot analysis of cell lysates from 293FT cells cotransduced with lentiviral vector (Lenti-Vect), lentiviruses carrying WT VE-cadherin (Lenti-VE-cad), retroviral vector (Retro-MiG), or retroviruses encoding p210-eGFP (Retro-MiG-p210). β-Catenin was densitometrically normalized to GAPDH with the control value set at 1.0, and all other values are shown relative to the control. (B) Immunoprecipitation of β-catenin was done using the above-mentioned cell lysates, and Western blots were probed with anti–P-Tyr (Phospho-Tyr102), anti–β-catenin, anti–phospho-Src, and anti-Src antibodies. IgG heavy chain served as the loading control. (C) DLR assays from the above 293FT cells transiently cotransfected with the phRL-TOP1 and phRL-TOP2 reporters and an internal pGL4.13-CMV-Luc2 firefly luciferase reporter. Data are shown as mean plus or minus SD (n = 3). The * denotes significant differences compared with vector controls (P < .01) based on the Student t test. (D) Western blot analysis of β-catenin stabilization from 293FT cells coexpressing VE-cadherin/eGFP-p210 or VE-cadherin/eGFP treated with 10 μM Src kinase inhibitor PP2, 10 μM Bcr-abl inhibitor imatinib mesylate, or both inhibitors for 16 hours. β-Catenin was densitometrically normalized to VE-cadherin with the untreated control value of expressing MiG vector alone set to 1.0. All other ratios are normalized to this control value. (E) VE-cadherin was immunoprecipitated from 293FT cells coexpressing VE-cadherin/eGFP-p210 or VE-cadherin/eGFP. The immunoprecipitates and cell lysates were loaded in parallel and probed with anti–phospho-Tyrosine (4G10) and VE-cadherin, c-Abl and eGFP antibodies. (F) β-Catenin immunoprecipitated either from 293FT expressing eGFP-210 or eGFP alone, or from Ph+ Sup-B15 or Ph− REH leukemic cells was in vitro labeled with recombinant ubiquitin and subjected to in vitro degradation assay as described in “In vitro poly-ubiquitination of β-catenin.”
Thus, consistent with the recent reports that Bcr-abl fusion protein promotes Src activation and that Bcr-abl mediates tyrosine phosphorylation and subsequent stabilization of β-catenin in Ph+ ALL cells,20,21 our data indicated that up-regulated Bcr-abl fusion protein contributes to constitutively active β-catenin activity by promoting VE-cadherin expression (physical stabilization) and subsequently converting VE-cadherin–stabilized β-catenin to the active form through Src-mediated tyrosine phosphorylation (functional stabilization). These data also suggest that tyrosine phosphorylation and serine/threonine phosphorylation may differentially influence the fate of β-catenin by affecting its subsequent ubiquitination.
Discussion
Compared with the LSC model of AML, the origin of ALL LSCs is less well characterized. Studies indicated that primitive HSCs were the target for ALL LSC transformation.22-24 In contrast, Castor et al25 showed that different genetic alterations may target different stages of hematopoiesis in ALL. The p210 Bcr-abl targeted primitive HSCs, whereas p185 and TEL-AML1 can transform CD34+CD19+ B-cell progenitors,25 raising the question whether LSCs for Ph+ ALL may also derive from committed B-cell progenitors.
Our data support the hypothesis that LSCs of B-cell Ph+ ALL can originate from committed progenitor cells. In the current study, we identified a CD34+CD38+CD19+Ph+/− ALL subpopulation with leukemic stem cell–like properties (Figure 1, 2). Formation of hematopoietic colonies (hemospheres) and cords, induction of endothelial sprouting, and reactivation of early stem cell genes in this CD34+CD38+CD19+ subset during LTCC are consistent with a leukemic stem cell phenotype (Figure 2). Expression of a panel of endothelial cell surface markers, including VE-cadherin, does not necessarily imply hemangioblast origin. Rather, it may represent one of the biologic consequences of Bcr-abl expression in this unique setting (Figure 6). In agreement with this possibility, a report showed that Bcr-abl fusion transcripts can be detected in endothelial cells derived from transplanted bone marrow cells from a patient with CML.26 This in vivo observation, together with our in vitro induction of endothelial differentiation of Ph+/VE-cadherin+ ALL cells (Figure 2), supports the conclusion that Bcr-abl alone may promote VE-cadherin expression (Figure 6) and contribute to vascular endothelium potential.26 Intriguingly, normal myeloid progenitors can also differentiate into endothelial cells,27 further supporting the notion that determination of endothelial fate is not restricted to the hemangioblast or HSC stage, but rather reflects the close developmental relation between the hematopoietic and vascular systems.
VE-cadherin expression in nonendothelial cells has been identified and characterized in 2 additional cell types to date. Increased VE-cadherin expression on cytotrophoblast stem cells was required for normal placentation and successful endovascular invasion.28 In addition, VE-cadherin was expressed in highly aggressive melanoma and contributed to vascular mimicry.29 Notable are the observations that gestational trophoblastic neoplasia and aggressive melanoma are highly metastatic with frequent central nervous system involvement.30,31 It is also widely recognized that, compared with acute nonlymphoblastic leukemia, non-Hodgkin lymphoma, or Hodgkin lymphoma, ALL is clinically manifested with a much higher frequency of central nervous system (CNS) involvement. Because nonendothelial cells with VE-cadherin expression identified so far share a common clinical feature, CNS involvement, it is tempting to speculate that VE-cadherin expression plays an important role in mediating invasion of Ph+/VE-cadherin+ ALL cells into the CNS.
Identification of the importance of the Bcr-abl/VE-cadherin/β-catenin axis and lack of reliance on Wnt initiated signals indicate that this subset of tumor cells can circumvent the requirement of exogenous Wnts for sustained self-renewal activity (Figures 4,5). This self-renewal autonomy is sustained by stabilized β-catenin in the presence of VE-cadherin expression (Figure 4) coincident with high Bcr-Abl kinase activity. Thus, these Ph+/VE-cadherin+ cells can bypass the de novo signaling cascade initialized by Wnt ligands for self-renewal and proliferation. Therefore, our data characterize a novel model in which the molecular basis underlying the self-renewal autonomy of Ph+ ALL LSCs can be further investigated.
Although Wnt/β-catenin–mediated self-renewal is similar in many tumor models, the epigenetic and genetic alterations imposed on the tumor by the microenvironment are diverse. Self-renewal autonomy conferred by intrinsically active β-catenin reflects one of the differences between normal hematopoietic stem cells and leukemic stem cells, because normal stem cells exquisitely rely on self-renewal signals from supportive niches,32 whereas cancer stem cells can somewhat escape this dependency. However, a lack of reliance by tumor cells on niche-derived signals does not indicate a loss of ability to respond to these important microenvironmental cues. In our model, bone marrow stromal cells modulate the β-catenin–mediated self-renewal capacity by up-regulating VE-cadherin and Bcr-abl expression (Figures 4,6). This observation is consistent with a report that up-regulation of Bcr-abl transcripts, followed by nuclear translocation of β-catenin, plays an important role during LSC transformation to CML blast crisis.33 Coexpression of VE-cadherin and Bcr-abl is unique to the subset of putative LSCs used in this model, with our data suggesting that microenvironmental factors remain influential in augmenting the self-renewal potential of specific LSCs.
In addition, recent studies indicated that conditional expression of constitutively active β-catenin in knock-in mice led to the exhaustion of the long-term hematopoietic stem cell pool and loss of stem cell–repopulating activity.34,35 This raised the question whether constitutively active β-catenin also impairs the plasticity of leukemic stem cells. Although β-catenin is constitutively active in LTCC Ph+VE-cadherin+ cells, in the presence of stromal cells, many early stem cell genes were reactivated in Ph+ ALL leukemic cells physically located in hemospheres (Figure 2). These early stem cell markers were previously shown to be expressed in primitive embryonic stem cells and serve as indicators of quiescence and pluripotency of embryonic stem cells.36 The observation that tumor cells expressed early stem cell markers in a specific anatomical configuration, with hemosphere structure initiation supported by physical attachment to bone marrow stromal cells, was striking. This observation suggests that stromal cell dependency distinguishes primitive leukemic stem cells from more differentiated progeny leukemic cells. Further, our data also suggest that leukemic cells in long-term coculture with stroma are highly heterogeneous, and in vitro LTCC may partially mimic the hierarchical differentiation of the hematopoietic stem/progenitor cells in the bone marrow microenvironment.
The concept of “reprogramming” differentiated cells to a stem cell–like phenotype has been addressed by several groups, including a recent report that the combination of 4 transcriptional factors, Oct-3/4, Sox2, c-myc, and klf4, can reprogram terminally differentiated mouse fibroblasts into pluripotent embryonic stem cells.37 Our data suggest that Ph+/VE-cadherin+ ALL cell gene expression profiles may be uniquely modified by stromal cells and support the hypothesis that formation of hemospheres with distinct stem cell marker expression may reflect an attempt by bone marrow stromal cells to balance the proliferating pool with the primitive, quiescent pool of LSCs. Therefore, unlike their normal hematopoietic counterparts,34,35 the LSC subpopulation of Ph+VE-cadherin+ ALL cells may not be exhausted even if constitutively active β-catenin is driving cell-cycle entry, because of stromal cell conversion of a pool of LSCs to a quiescent state. Thus, self-renewal autonomy and stromal cell dependence of Ph+VE-cadherin+ ALL cells may be integrated in a way that positions them to contribute to the relapse of disease through characteristics they share with normal HSCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Brett Hall of Ohio State University for kindly providing Ds-Red labeled Sup-B15 cells, and the additional investigators noted in the text for providing valuable cell lines and constructs.
This work was supported by National Institutes of Health grants R01 HL056888 and NCRR16440 (L.F.G.).
National Institutes of Health
Authorship
Contribution: L.W. and L.F.G. designed the research; L.W., H.O., and J.F., performed the research; L.W. and H.O. analyzed the data; L.W. and L.F.G. prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laura F. Gibson, PO Box 9214, Department of Pediatrics, School of Medicine, West Virginia University, Morgantown, WV 26506; e-mail: lgibson@hsc.wvu.edu