Abstract
The modes of action of intravenous immunoglobulins (IVIgs) in exerting their immunomodulatory properties are broad and not fully understood. IVIgs can modulate the function of various immune cells, including suppressing the capacity of dendritic cells (DCs) to stimulate T cells. In the present study, we showed that DCs matured in the presence of IVIgs (IVIg-DCs) activated NK cells, and increased their interferon-γ production and degranulation. The activated NK cells induced apoptosis of the majority of IVIg-DCs. In consequence, only in the presence of NK cells, IVIg-DCs were 4-fold impaired in their T-cell priming capacity. This was due to NK-cell–mediated antibody-dependent cellular cytotoxicity (ADCC) to IVIg-DCs, probably induced by IgG multimers, which could be abrogated by blockade of CD16 on NK cells. Furthermore, IVIg-DCs down-regulated the expression of NKp30 and KIR receptors, and induced the generation of CD56brightCD16−CCR7+ lymph node–type NK cells. Our results identify a novel pathway, in which IVIgs induce ADCC of mature DCs by NK cells, which downsizes the antigen-presenting pool and inhibits T-cell priming. By influencing the interaction between DCs and NK cells, IVIgs modulate the ability of the innate immunity to trigger T-cell activation, a mechanism that can “cool down” the immune system at times of activation.
Introduction
Intravenous immunoglobulins (IVIgs) are pharmaceutical preparations of human IgG purified from pools of plasma of thousands of donors. For decades IVIgs have been established as treatment of autoantibody-mediated1 and T-cell–mediated inflammatory disorders.2 The distribution of IgG subclasses in IVIgs is comparable with that of IgG in normal human serum, however, unlike IgG purified from a single individual, therapeutic IVIg preparations contain substantial amounts of IgG dimers and traces of multimers, due to the idiotype–anti-idiotype complex formation between IgG molecules from different individuals.3,4 In general, IVIgs were shown to be effective in conditions in which the immune system is hyperactive, but still the mechanisms of action by which IVIgs correct immune dysregulation are not fully understood. Various reports showed that the mode of actions of IVIgs is multifaceted and complex, involving interference with different components of the immune system. Clinical and immunologic improvements induced by IVIg treatment are reported to be profound and to extend the half-life of infused IgG, suggesting that IVIgs can modify cellular immune reactivity for prolonged periods.1,5
Recently, we observed that hyperimmune IVIgs against hepatitis B surface antigen (HBs) protect against acute rejection after liver transplantation, indicating that IVIg treatment can modulate the T-cell–mediated immune response against alloantigens.6 We and others found that in vitro both anti-HBs IVIgs and nonspecific IVIgs are able to suppress T-cell proliferation and cytokine production, and to impair the allogeneic T-cell stimulatory capacity of monocyte-derived and blood-derived dendritic cells (DCs),6-9 demonstrating that IVIgs can suppress T-cell responses both during the priming phase as well as in the effector phase. The importance of DCs as a cellular target of IVIgs in vivo was recently shown in a murine model of immune thrombocytopenic purpura (ITP), in which treatment with IVIgs could be replaced by adoptive transfer of IVIg-treated DCs.10 With regard to the mechanism by which IVIgs affect DC function, we found that the decreased T-cell stimulatory capacity of IVIg-treated DCs (IVIg-DCs) was associated with induction of cell death in mature DCs. Interestingly, IVIg treatment itself did not induce DC death directly, as the increased death of IVIg-DCs occurred only when cultured with other immune cells (ie, T cells and natural killer [NK] cells).9 As activated NK cells are capable of killing DCs in a number of circumstances,11,12 we hypothesized that NK cells may induce apoptosis of IVIg-DCs during the initial phase of DC–NK-cell encounter (ie, before the T-cell activation).
Interactions between DCs and NK cells have been documented in a variety of settings, shedding light on the complexity of the bidirectional interaction between these 2 cell types. Bajenoff et al showed that NK cells are present in the medulla and the paracortex of lymph nodes, where they closely interact with DCs. Upon receiving an inflammatory signal, NK cells interact with DCs and regulate colocalized T-cell responses.13 Cross talk between DCs and NK cells can result in lysis, inhibition, or maturation of DCs by NK cells, and reciprocally, DCs can activate or inhibit NK-cell functions. The final outcome of DC–NK-cell interaction depends on the conditions in which both cell types encounter each other,12,14 and will subsequently determine the development of the following adaptive immune response.
In this study, we investigated the mechanism by which IVIgs modulate the interaction between DCs and NK cells, and how this consequentially shapes T-cell priming. Our observations form the basis of a model to clarify how administration of IVIgs may “cool down” hyperactivity of the cellular immune system for extended periods.
Materials and methods
Approval for this study was obtained from the review board of the Medische Ethische Toetsings Commissie (METC) of the Erasmus MC–University Medical Center. Informed consent was obtained in accordance with the Declaration of Helsinki.
Reagents
Human IVIgs (Intraglobin CP) were a kind gift from Biotest Pharma (Dreieich, Germany), and the humanized monoclonal antibody trastuzumab was kindly provided by Roche Pharma (Mijdrecht, The Netherlands). Both preparations were dialyzed against large volumes of culture medium (RPMI) at 4°C using Slide-A-lyzer gamma-irradiated dialysis cassettes (Pierce, Rockford, IL) to remove stabilizing agents and to obtain neutral pH. After dialysis, IgG concentration was determined by the Tina-quant immuno-turbidimetric assay (Roche Diagnostics, Mannheim, Germany). In all experiments, IVIgs were used in a concentration of 10 mg/mL (0.06 M). This is similar to increments in serum IgG concentration in patients treated with IVIgs at 1 to 2 g/kg for autoimmune disorders.15-17 To detect binding of IVIgs, biotinylated IVIgs or rabbit F(ab′)2 anti–human IgG-FITC (DAKO Cytomation, Glostrup, Denmark) was used. The biotinylation was performed using D-biotinoyl-ϵ-aminocaproic acid N-hydroxysuccinimide ester (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. To exclude that the suppressive effects of IVIgs were due merely to an elevated protein concentration in the cultures, human serum albumin (HSA; Sanquin Research, Amsterdam, the Netherlands) was used as a negative control.
Isolation of immune cells
DCs, NK cells, and T cells were purified from fresh heparinized blood of healthy individuals. After Ficoll density gradient separation and depletion of B cells with CD19-conjugated immunomagnetic beads and separation over large depletion (LD) columns (Miltenyi Biotec, Bergisch Gladbach, Germany), DCs were isolated by positive selection with PE-conjugated anti-CD1c mAb and anti-PE immunomagnetic beads using Medium Separation-columns, as described previously.6 The purity of DCs (defined as CD1c+CD20− cells) as analyzed by flow cytometry was higher than 95%.
NK cells were negatively selected by the NK-cell isolation Kit (Miltenyi Biotec) using LD columns. The purity of NK cells (defined as CD56+CD3− cells) as analyzed by flow cytometry was higher than 95%.
T cells were enriched by immunomagnetic depletion of B cells, monocytes, DCs, and NK cells. From the same healthy blood, donor T cells were also isolated without NK-cell depletion. The cells were labeled with CD19-conjugated immunomagnetic beads, PE-conjugated anti-CD1c mAb (both from Miltenyi Biotec), CD14-PE and/or CD56-PE (both from BD Biosciences, Erembodegem, Belgium), followed by incubation with anti-PE immunomagnetic beads and separation over LD columns. The purified T-cell preparations contained 92% (± 4%) CD3+ cells and less than 0.3% CD56+ cells. Contaminating cells consisted of granulocytes. The T-cell preparation from which NK cells were not depleted contained 80% CD3+CD56− T cells and 12% CD3−CD56+ NK cells.
Effects of IVIgs and NK cells on allogeneic T-cell stimulatory capacity of DCs
If not otherwise mentioned, in all experiments with DCs, immature human blood DCs were stimulated to mature with TNFα (25 ng/mL) and IL-1β (50 ng/mL) (both from Strathmann Biotech, Hanover, Germany) in RPMI supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), 10% FCS (Hyclone, Logan, UT), and granulocyte macrophage colony-stimulating factor (GM-CSF, 500 U/mL; Leucomax, Arnhem, the Netherlands) for 18 hours in the absence or presence of IVIgs or HSA.
To determine the effects of IVIgs on the acquisition of allogeneic T-cell stimulatory capacity of DCs, and the role of NK cells in this process, different numbers of immature blood DCs (5 × 103, 2.5 × 103, 1.25 × 103, and 0.75 × 103 cells per well of a 96-well flat bottom plate) were stimulated with TNFα and IL-1β for 18 hours in the absence (CTRL-DCs) or presence (IVIg-DCs) of 10 mg/mL IVIgs. The next day, DCs were washed, and either allogeneic T cells that contained 12% NK cells (ie, 1.2 × 105 plus T cells 0.18 × 105 NK cells) or 1.2 × 105 pure allogeneic T cells were added. In all experiments with allogeneic T cells, the one T-cell and one T + NK-cell batch obtained from the same healthy blood donor were used. For addition of autologous NK cells (Figure 5D), 0.18 × 105 NK cells from the same donor as the DCs combined with 1.2 × 105 allogeneic T cells were added per well. After 5 days, proliferation was assessed by determination of the incorporation of 0.5 μCi (0.0185 MBq) [3H] thymidine (Radiochemical Center, Amersham, United Kingdom) for 18 hours. In addition, to determine the effects of IVIgs on T-cell and NK-cell expansion separately, T cells (with or without NK cells) were labeled with 2 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) per 5 × 106 cells for 10 minutes at 37°C. Labeled T cells with or without NK cells from the same donor were cultured with allogeneic CTRL-DCs and IVIg-DCs. After 6 days, T- and NK-cell divisions were analyzed by flow cytometry after labeling the cells with CD3-PE and CD56–allophycocyanin (APC) (both from Beckman Coulter Immunotech, Marseille, France). CFSE flow cytometry data were analyzed by ModFit software version 3.0 (Verity Software House, Topsham, ME). The software calculated the proliferation index (PI), which indicates the extent of cell expansion and the precursor frequency (% prec), which is the percentage of cells that underwent at least one division.
To block the FcγRIII on NK cells, we added CD16 blocking antibody (clone 5D2; 10 μg/mL18 ), which was kindly provided by Dr M. de Haas (Sanquin Research) to the cultures.
Detection of binding of IVIgs to DCs, DC apoptosis, and NK-cell activation
To detect binding of IVIgs to DCs, DCs were matured in the presence or absence of biotinylated IVIgs for 18 hours in Dulbecco modified Eagle medium (DMEM) supplemented with ultraglutamine with 4.5 g/L glucose (BioWhittaker, Verviers, Belgium), penicillin, streptomycin, 10% FCS, and GM-CSF, TNFα, and IL-1β. DMEM was used instead of RPMI, since RPMI contains biotin. After DCs were washed extensively to remove all nonbound IVIg-biotin, biotinylated IVIgs on the DC membrane were detected using streptavidin-APC (BD Biosciences, Erembodegen, Belgium). To detect internalization of IVIg-biotin, first, surface-bound biotinylated IVIgs were detected using streptavidin-PerCP (BD Biosciences). Subsequently, residual-free biotinylated IVIgs on the cell surface were blocked using biotin-blocking reagent (DAKO Cytomation), DCs were fixed and permeabilized with Intraprep permeabilization reagents (Beckman Coulter Immunotech), and intracellular IVIg-biotin was detected using streptavidin-APC. Alternatively, FITC-conjugated rabbit anti–human IgG F(ab)2 was used to detect binding of nonbiotinylated IVIgs on the DC surface.
After maturation, the culture medium was aspirated, DCs were washed 2 times with culture medium, and 2.4 × 105 allogeneic or autologous NK cells were added. The DC/NK ratio was 1:6, which is between the ratio used in the mixed lymphocyte reaction performed with 5000 and 2500 DCs (Figure 1A). To determine apoptosis of stimulated DCs in these DC-NK cocultures, the number of active caspase-3–expressing CD1c+ DCs was determined after 8-hour incubation with allogeneic NK cells by intracellular labeling with anti–active caspase-3-FITC mAb (BD Pharmingen) using Intraprep permeabilization reagents, after membrane labeling with PE-conjugated anti-CD1c mAb. Secondly, cell death of DCs at 18 hours of coculture of DCs with NK cells was detected by annexin V–APC staining combined with 7-AAD uptake in CD1c+ DC (both BD Biosciences, Heidelberg, Germany) and analyzed by flow cytometry. In addition, we determined the absolute numbers of viable DCs in the cultures at 18 and 48 hours after coculture of mature DCs with allogeneic NK cells by adding a fixed number of Calibrite beads (BD Biosciences) to the cells and determining the ratio of 7-AAD− DCs to beads by flow cytometry. Absolute numbers of viable DCs were calculated by multiplying this ratio by the absolute number of beads added to the cells.19
After 48 hours of DC–NK-cell coculture, supernatants were collected for cytokine production, and NK-cell activation was established using CD56-PE, CD69-APC, and CD25-APC (all from BD Biosciences). Degranulation of NK cells was determined in 6-hour cocultures of NK cells and DCs by adding PE-conjugated CD107a mAb (BD Pharmingen) to the cultures, according to the protocol described in the literature.20,21 To detect spontaneous degranulation, a control sample of NK cells without DCs was included in all experiments. IFN-γ concentration in the supernatants was quantified by enzyme-linked immunosorbent assay (ELISA; U-CyTech, Utrecht, the Netherlands).
Effect of IVIg-DCs on NK-cell phenotype
To verify whether IVIg-DCs promote phenotypical changes of NK cells, we cultured 104 DCs with 4.8 × 105 allogeneic T and 1.2 × 106 NK cells (ratio DC/T/NK is 1:48:12), and after 5 days we determined the expression of FcγRIII (CD16), natural cytotoxicity receptors (NCRs), killer immunoglobulin-like receptors (KIRs), and lymph node homing chemokine receptors using CD16-PE (clone 3G8), NKG2A-PE, NKp30-PE, CD158a-APC, CD158b-APC (all from Beckman Coulter Immunotech), CXCR3-APC, and CCR7-FITC (both from R&D systems, Minneapolis, MN) on CD56+CD3− cells. We have checked whether IVIgs prevented binding of the 3G8 mAb to NK cells by preincubating NK cells with 10 mg/mL IVIgs at 4°C, and found that 3G8 binding was not affected by IVIgs.
Statistical analysis
In each individual experiment, proliferation and cytokine production were tested in triplicate, from which means were calculated. In the results, means with SD from independent experiments are depicted. Statistical analyses were performed by the Wilcoxon test for paired data or the Student t test for paired data using software package SPSS version 10.1 (SPSS, Chicago, IL) as indicated in the legend. A 2-sided P less than .05 was considered as indicating significant difference.
Results
Impaired allogeneic T-cell priming by IVIg-DCs occurs only in the presence of NK cells
To study whether NK cells were involved in reduction of the capacity of IVIg-treated DCs (IVIg-DCs) to prime allogeneic T cells, we matured DCs by addition of IL-1β and TNF-α in the presence or absence of 10 mg/mL IVIgs for 18 hours. Then the additions were washed out, and either allogeneic T cells, which contained 12% NK cells, or pure allogeneic T cells, where the NK-cell contamination level was less than 0.3%, were added. When IVIg-DCs were cocultured with allogeneic T and NK cells, their capacity to prime allogeneic T cells was 4-fold impaired (P < .05, N = 4). However, this effect was not observed when NK cells were absent in the cultures (Figure 1A). Using CFSE-labeled cells, it was confirmed that the effect of IVIg treatment on the allogeneic T-cell stimulatory capacity of DCs is dependent on the presence of NK cells, and that the reduced [3H] thymidine incorporation observed in Figure 1A indeed reflected lowered T-cell proliferation (Figure 1B,C).
Impaired allogeneic T-cell priming by IVIg-DCs occurs only in the presence of NK cells. (A) Indicated numbers of immature blood DCs were stimulated with TNFα and IL-1β for 18 hours in the absence (CTRL-DC, —) or presence of 10 mg/mL IVIgs (IVIg-DC, –). The next day, DCs were washed, and allogeneic T cells, which contained 12% NK cells, or the same number of allogeneic T cells without NK cells were added. Proliferation was assessed after 5 days by determination of the incorporation of [3H] thymidine (N = 4; *P < .05, **P < .01, paired Student t test). Error bars are SD. (B) Purified allogeneic T cells and NK cells labeled with CFSE were added and subsequently cultured with CTRL-DCs (left) or IVIg-DCs (right) at a DC/T/NK ratio of 1:48:12. At day 6 of coculture, cells were labeled with CD3-PE and CD56-APC, and the CFSE dilution profile of CD3+CD56− T cells was analyzed using ModFit software. CFSE analysis was also performed on CD56+CD3− NK cells, which is shown in Figure 7A separately. (C) The same experiment, as depicted in panel B, but CFSE-labeled allogeneic T cells without NK cells, were cultured with CTRL-DCs (left) or IVIg-DCs (right) at a DC/T ratio of 1:48. Proliferation index (PI) and percentage of precursor T cells (% Prec) depicted in panels B,C are means plus or minus SD from 4 independent experiments.
Impaired allogeneic T-cell priming by IVIg-DCs occurs only in the presence of NK cells. (A) Indicated numbers of immature blood DCs were stimulated with TNFα and IL-1β for 18 hours in the absence (CTRL-DC, —) or presence of 10 mg/mL IVIgs (IVIg-DC, –). The next day, DCs were washed, and allogeneic T cells, which contained 12% NK cells, or the same number of allogeneic T cells without NK cells were added. Proliferation was assessed after 5 days by determination of the incorporation of [3H] thymidine (N = 4; *P < .05, **P < .01, paired Student t test). Error bars are SD. (B) Purified allogeneic T cells and NK cells labeled with CFSE were added and subsequently cultured with CTRL-DCs (left) or IVIg-DCs (right) at a DC/T/NK ratio of 1:48:12. At day 6 of coculture, cells were labeled with CD3-PE and CD56-APC, and the CFSE dilution profile of CD3+CD56− T cells was analyzed using ModFit software. CFSE analysis was also performed on CD56+CD3− NK cells, which is shown in Figure 7A separately. (C) The same experiment, as depicted in panel B, but CFSE-labeled allogeneic T cells without NK cells, were cultured with CTRL-DCs (left) or IVIg-DCs (right) at a DC/T ratio of 1:48. Proliferation index (PI) and percentage of precursor T cells (% Prec) depicted in panels B,C are means plus or minus SD from 4 independent experiments.
IVIgs are present on the surface and in the cytoplasm of DCs after maturation and increase DC susceptibility for NK-cell–mediated killing
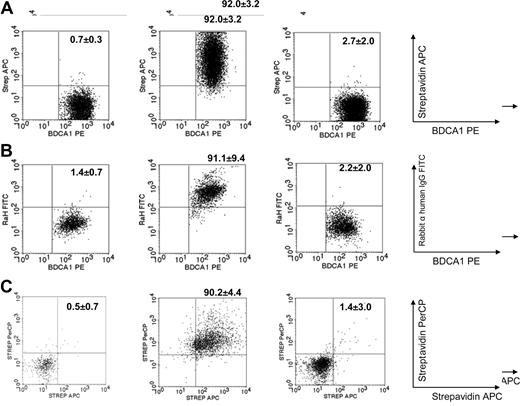
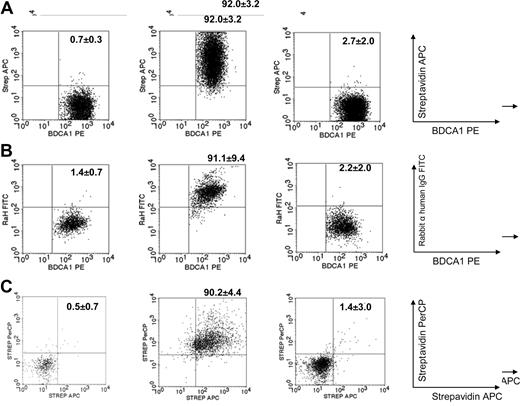
Using biotinylated IVIgs allowed clear detection of IVIgs in the cytoplasm and on the surface of DCs with streptavidin fluorochromes. Binding of IVIg-biotin was detected on the surface of 92% (± 3%) of DCs matured in the presence of biotinylated IVIgs (N = 3). Untreated DCs (CTRL-DCs) or DCs matured in the presence of HSA (HSA-DCs) did not bind streptavidin-APC (Figure 2A). A second method using FITC-conjugated rabbit anti–human IgG F(ab)2 to detect IVIg binding on DCs showed equivalent findings, as 91% (± 9%) of the IVIg-DCs were positively stained (N = 4) (Figure 2B).
Presence of IVIgs on the surface and in the cytoplasm of DCs after maturation. (A) DCs were stimulated for 18 hours in the absence (left plot) or presence of biotinylated IVIgs (middle plot) or HSA (right plot). Using streptavidin-APC, binding of IVIg-biotin was detected on 92% (± 3%) of the surface of IVIg-DCs after maturation (N = 3). (B) DCs were stimulated for 18 hours in the absence (left plot) or presence of IVIgs (middle plot) or HSA (right plot). Rabbit anti–human IgG-FITC F(ab)2 was used to detect IVIg binding on the DC membrane. This showed equivalent results (N = 4). (C) To determine internalization of IVIgs during maturation, biotinylated IVIgs on the DC surface were detected by streptavidin-PerCP. Thereafter, residual-free biotinylated IVIgs on the cell surface were blocked using biotin-blocking reagent, DCs were fixed and permeabilized, and intracellular biotinylated IVIgs were detected using streptavidin-APC. We observed that IVIgs were present on the surface as well in the cytoplasm of the DCs (N = 3). Numbers on plots are percentage positive cells (± SD).
Presence of IVIgs on the surface and in the cytoplasm of DCs after maturation. (A) DCs were stimulated for 18 hours in the absence (left plot) or presence of biotinylated IVIgs (middle plot) or HSA (right plot). Using streptavidin-APC, binding of IVIg-biotin was detected on 92% (± 3%) of the surface of IVIg-DCs after maturation (N = 3). (B) DCs were stimulated for 18 hours in the absence (left plot) or presence of IVIgs (middle plot) or HSA (right plot). Rabbit anti–human IgG-FITC F(ab)2 was used to detect IVIg binding on the DC membrane. This showed equivalent results (N = 4). (C) To determine internalization of IVIgs during maturation, biotinylated IVIgs on the DC surface were detected by streptavidin-PerCP. Thereafter, residual-free biotinylated IVIgs on the cell surface were blocked using biotin-blocking reagent, DCs were fixed and permeabilized, and intracellular biotinylated IVIgs were detected using streptavidin-APC. We observed that IVIgs were present on the surface as well in the cytoplasm of the DCs (N = 3). Numbers on plots are percentage positive cells (± SD).
To determine whether DCs internalized IVIgs during maturation, we first detected biotinylated IVIgs on the DC surface by streptavidin-PerCP. Thereafter, intracellular biotinylated IVIgs were detected using streptavidin-APC (Figure 2C). We observed that 90% (± 4%) of DCs stained for both streptavidin-PerCP and streptavidin-APC, indicating that IVIgs were present on the surface as well in the cytoplasm of the DCs (N = 3) (Figure 2C).
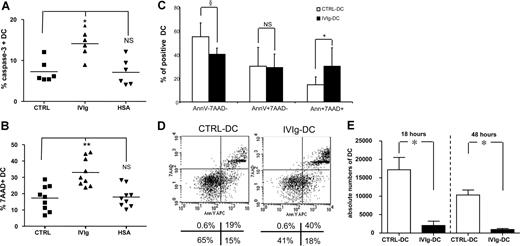
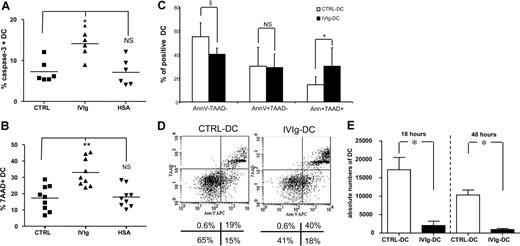
When allogeneic NK cells were cultured with IVIg-DCs, the NK cells induced apoptosis of IVIg-DCs, as shown by increased intracellular expression of active caspase 3 (CTRL-DCs: 7% ± 2%, IVIg-DCs: 14% ± 3%, HSA-DCs: 7% ± 3%, P < .01, N = 6) after 8 hours of DC–NK-cell encounter (Figure 3A). Subsequently, after 18 hours of coculture we observed increased DC death, as indicated by enhanced 7-AAD uptake in IVIg-DCs (CTRL: 17% ± 8%, IVIgs: 33% ± 9%, HSA: 18% ± 6%, P < .01, N = 9) (Figure 3B). The increased DC death was not the result of allogeneic differences, as autologous NK cells were also able to induce enhanced killing of IVIg-DCs (CTRL: 16% ± 9%, IVIgs: 35% ± 10%, HSA: 17% ± 8% 7-AAD+ DCs, P < .05, N = 4). In addition, annexin V staining combined with 7-AAD was performed. The proportion of annexin V+7-AAD+ cells was increased in the IVIg-DC population (CTRL-DCs: 15% ± 6%, IVIg-DCs: 30% ± 15%, P < .05, N = 6), while the proportion of annexin V−7-AAD− IVIg-DCs was reduced compared with CTRL-DCs (CTRL-DCs: 55% ± 12%, IVIg-DCs: 40% ± 5%, P < .08, N = 6) (Figure 3C-D). To exclude that the effects of IVIg treatment of DCs were specific for DCs stimulated with proinflammatory cytokines, we repeated the experiments with DCs stimulated with LPS. Indeed, IVIg treatment during DC stimulation with LPS resulted in equivalent increases of DC death after addition of NK cells (data not shown).
IVIg treatment increases the susceptibility of matured DCs for NK-mediated killing. (A) After maturation with IL-1β and TNF-α, DCs were cultured with allogeneic NK cells at the ratio of 1:6. Eight hours thereafter, intracellular expression of active caspase-3 in CTRL-DCs (■), IVIg-DCs (▴), and HSA-DCs (▾) was determined (N = 6; Wilcoxon test for paired data, *P < .05 compared with CTRL-DCs; NS indicates not significant). (B) After 18 hours of coculture, DC death was monitored by 7-AAD uptake in CTRL-DCs (■), IVIg-DCs (▴), and HSA-DCs (▾) (N = 9; Wilcoxon test for paired data, **P < .01 compared with CTRL-DCs; NS indicates not significant). (C,D) After 18 hours of coculture of matured DCs with allogeneic NK cells, DC apoptosis was monitored by annexin V staining combined with 7-AAD. (N = 6; Wilcoxon test for paired data, §P < .08, *P < .05 compared with CTRL-DCs; NS indicates not significant.) Error bars are SD. Panel D shows plots of 1 representative experiment of 6 experiments. Numbers below panel D are the percentages of cells in each quadrant. (E) After 18 and 48 hours of coculture of 40 000 matured DCs with allogeneic NK cells (DC/NK ratio, 1:6), cells were harvested from the cultures and fixed numbers of Calibrite beads were added. Absolute numbers of viable DCs were calculated by determining the ratio of 7-AAD− DCs to beads and then multiplying this ratio by the number of beads in the tube. Data are depicted as mean with SE (N = 7; *P < .05, Wilcoxon test for paired data).
IVIg treatment increases the susceptibility of matured DCs for NK-mediated killing. (A) After maturation with IL-1β and TNF-α, DCs were cultured with allogeneic NK cells at the ratio of 1:6. Eight hours thereafter, intracellular expression of active caspase-3 in CTRL-DCs (■), IVIg-DCs (▴), and HSA-DCs (▾) was determined (N = 6; Wilcoxon test for paired data, *P < .05 compared with CTRL-DCs; NS indicates not significant). (B) After 18 hours of coculture, DC death was monitored by 7-AAD uptake in CTRL-DCs (■), IVIg-DCs (▴), and HSA-DCs (▾) (N = 9; Wilcoxon test for paired data, **P < .01 compared with CTRL-DCs; NS indicates not significant). (C,D) After 18 hours of coculture of matured DCs with allogeneic NK cells, DC apoptosis was monitored by annexin V staining combined with 7-AAD. (N = 6; Wilcoxon test for paired data, §P < .08, *P < .05 compared with CTRL-DCs; NS indicates not significant.) Error bars are SD. Panel D shows plots of 1 representative experiment of 6 experiments. Numbers below panel D are the percentages of cells in each quadrant. (E) After 18 and 48 hours of coculture of 40 000 matured DCs with allogeneic NK cells (DC/NK ratio, 1:6), cells were harvested from the cultures and fixed numbers of Calibrite beads were added. Absolute numbers of viable DCs were calculated by determining the ratio of 7-AAD− DCs to beads and then multiplying this ratio by the number of beads in the tube. Data are depicted as mean with SE (N = 7; *P < .05, Wilcoxon test for paired data).
Furthermore, after 18 and 48 hours of coculture with allogeneic NK cells, we have determined the absolute numbers of viable 7-AAD− DCs in the cultures by flow cytometry after addition of known numbers of Calibrite beads. After coculture with NK cells, the absolute numbers of viable IVIg-DCs were profoundly reduced compared with CTRL-DCs (18 hours: IVIg-DCs: 2046 ± 1173, CTRL-DCs: 17 181 ± 1266; 48 hours: IVIgs: 968 ± 266, CTRL: 10 318 ± 1363, P < .05, N = 7) (Figure 3E).
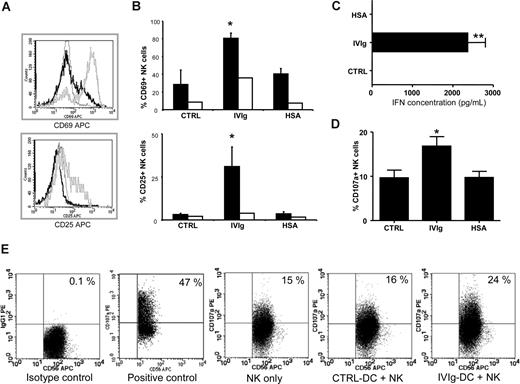
IVIg-DCs trigger NK-cell activation, IFN-γ production, and degranulation
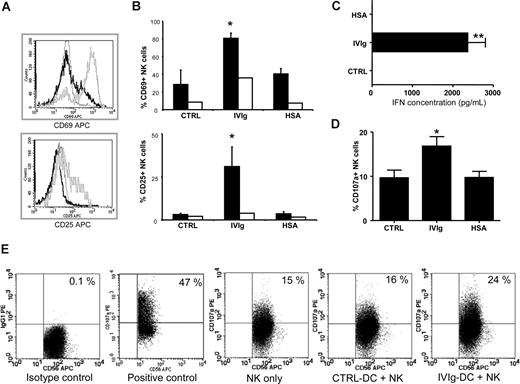
To investigate the effect of IVIg-DCs on NK-cell activation and cytokine production, allogeneic NK cells were cocultured with IVIg-DCs, HSA-DCs, or CTRL-DCs. After 48 hours of coculture, we observed that IVIg-DCs promoted activation of allogeneic NK cells as determined by CD69 expression (CTRL: 29% ± 16%, IVIgs: 81% ± 6%, and HSA: 41% ± 6%, P < .01, N = 6) and CD25 expression (CTRL: 3% ± 0.8%, IVIgs: 31% ± 11%, and HSA: 4% ± 1%, P < .01, N = 5) on NK cells (Figure 4A-B). In contrast, 48-hour treatment of NK cells with 10 mg/mL IVIgs in absence of DCs had marginal activating effects on NK cells, showing that full NK-cell activation requires both DCs and the IVIg treatment of DCs prior to coculture with NK cells. Moreover, supernatants collected at 48 hours from the cocultures of IVIg-DCs and NK cells contained high levels of interferon γ (IFN-γ) (IVIg-DCs and NK cells: 2.4 ± 0.2 ng/mL), while in the supernatants of NK cells cultured with CTRL-DCs or HSA-DCs, no IFN-γ could be detected (N = 7) (Figure 4C). IL-10 production was not detectable in any of the collected supernatants. Again, the increased activation and cytokine production of NK cells were not due to allogeneic differences, since experiments performed with autologous NK cells showed equivalent results (data not shown).
IVIg-DCs trigger NK-cell activation, IFN-γ production, and degranulation. (A) IVIg-DCs, HSA-DCs, or CTRL-DCs were cultured for 48 hours with allogeneic NK cells (ratio DC/NK was 1:6) after which CD69 (top plot) and CD25 expression (bottom plot) on CD56+ cells were determined. Depicted are NK cells activated by IVIg-DCs (gray histogram) and by CTRL-DCs (black histogram), and staining with an irrelevant isotype control mAb of NK cells activated by IVIg-DCs (dotted histogram). Similar NK activation was observed when DC/NK ratio was changed to 1:3 or 1:1 (data not shown). (B) IVIg-DCs, HSA-DCs, or CTRL-DCs were washed to remove additives, and cultured for 48 hours with allogeneic NK cells (■). CD69 expression (N = 6; Wilcoxon test for paired data, *P < .05) and CD25 expression (N = 5; Wilcoxon test for paired data, *P < .05) on NK cells were significantly enhanced after coculture with IVIg-DCs. Treatment of NK cells with 10 mg/mL IVIgs or HSA in absence of DCs (□) had only minor activating effects on NK cells. (C) IFN-γ concentration was determined in cell-free supernatants of DC-NK cocultures. In the supernatants of NK cells stimulated with CTRL-DCs or HSA-DCs, no IFN-γ could be detected, while in the supernatants of NK cells stimulated with IVIg-DCs, high IFN-γ levels were detected (N = 7; Wilcoxon test for paired data, *P < .01). (D) Expression of the lytic granule membrane protein CD107a on the NK-cell surface after 6 hours of coculture of matured allogeneic CTRL-DCs, IVIg-DCs, or HSA-DCs (N = 5; Wilcoxon test for paired data, *P < .05). Error bars in panels B-D are SD. (E) Representative dot plots showing CD107a expression on NK cells cultured for 6 hours without DCs (negative control), NK cells stimulated with PMA and ionomycin (2.5 μg/mL and 0.5 μg/mL, respectively) (positive control), NK cells cocultured with CTRL-DCs, and NK cells cocultured with IVIg-DCs. Numbers on plots are the percentage of cells in that quadrant.
IVIg-DCs trigger NK-cell activation, IFN-γ production, and degranulation. (A) IVIg-DCs, HSA-DCs, or CTRL-DCs were cultured for 48 hours with allogeneic NK cells (ratio DC/NK was 1:6) after which CD69 (top plot) and CD25 expression (bottom plot) on CD56+ cells were determined. Depicted are NK cells activated by IVIg-DCs (gray histogram) and by CTRL-DCs (black histogram), and staining with an irrelevant isotype control mAb of NK cells activated by IVIg-DCs (dotted histogram). Similar NK activation was observed when DC/NK ratio was changed to 1:3 or 1:1 (data not shown). (B) IVIg-DCs, HSA-DCs, or CTRL-DCs were washed to remove additives, and cultured for 48 hours with allogeneic NK cells (■). CD69 expression (N = 6; Wilcoxon test for paired data, *P < .05) and CD25 expression (N = 5; Wilcoxon test for paired data, *P < .05) on NK cells were significantly enhanced after coculture with IVIg-DCs. Treatment of NK cells with 10 mg/mL IVIgs or HSA in absence of DCs (□) had only minor activating effects on NK cells. (C) IFN-γ concentration was determined in cell-free supernatants of DC-NK cocultures. In the supernatants of NK cells stimulated with CTRL-DCs or HSA-DCs, no IFN-γ could be detected, while in the supernatants of NK cells stimulated with IVIg-DCs, high IFN-γ levels were detected (N = 7; Wilcoxon test for paired data, *P < .01). (D) Expression of the lytic granule membrane protein CD107a on the NK-cell surface after 6 hours of coculture of matured allogeneic CTRL-DCs, IVIg-DCs, or HSA-DCs (N = 5; Wilcoxon test for paired data, *P < .05). Error bars in panels B-D are SD. (E) Representative dot plots showing CD107a expression on NK cells cultured for 6 hours without DCs (negative control), NK cells stimulated with PMA and ionomycin (2.5 μg/mL and 0.5 μg/mL, respectively) (positive control), NK cells cocultured with CTRL-DCs, and NK cells cocultured with IVIg-DCs. Numbers on plots are the percentage of cells in that quadrant.
To elucidate whether IVIg-DCs stimulate NK-cell degranulation, we determined the expression of the lytic granule membrane protein CD107a on the NK-cell surface. Approximately 10% of NK cells cultured without DCs spontaneously expressed CD107a, and CTLR-DCs did not induce degranulation above this background. We observed an increased surface expression of CD107a on NK cells only after they had been cocultured with IVIg-DCs (CTRL-DCs: 10% ± 4%, IVIg-DCs: 17% ± 5%, HSA-DCs: 10% ± 3%, P < .05, N = 5) (Figure 4D). The enhanced degranulation of lytic granules by NK cells after coculture with IVIg-DCs supports the conclusion that NK cells are responsible for the enhanced killing of IVIg-DCs.
Impaired allogeneic T-cell priming is due to ADCC of IVIg-DCs by NK cells
To investigate whether NK cells recognize IVIg-treated DCs via their FcγRIII (CD16), a CD16-blocking antibody (clone 5D2) was added into DC–NK-cell cultures. The increased NK-cell–mediated killing of IVIg-DCs, as assessed by 7-AAD uptake in DCs, was significantly reduced from 37% (± 7%) to 28% (± 3%) of CD1c+ DCs (P < .05, N = 5) when the CD16-blocking antibody was added, while CD16 blockade had no effect on NK-cell–mediated lysis of CTRL-DCs and HSA-DCs (Figure 5A). In addition, by blocking CD16, more viable IVIg-DCs were present after coculture with NK cells (Figure 5B). This indicates that the increased NK-cell–mediated apoptosis of IVIg-DCs by NK cells is largely due to FcγRIII-mediated antibody-dependent cellular cytotoxicity (ADCC).
Impaired allogeneic T-cell priming is due to ADCC of IVIg-DCs by NK cells. (A) Matured CTRL-DCs, IVIg-DCs, or HSA-DCs were cultured for 18 hours with allogeneic NK cells (ratio, 1:6) with (black bars) or without (white bars) 10 μg/mL CD16-blocking antibody (5D2). DC death was determined by 7-AAD uptake. The increased death of IVIg-DCs was abrogated by blocking the FcγRIII on NK cells (N = 5; Wilcoxon test for paired data, *P < .05; NS indicates not significant). (B) Matured CTRL-DCs (□) and IVIg-DCs (■) were cultured for 18 hours with allogeneic NK cells (ratio, 1:6) with or without 10 μg/mL 5D2 antibody. Absolute numbers of viable DCs were calculated by determining the ratio of 7-AAD− DCs to detected beads and then multiplying this ratio by the number of beads in the tube. Data are depicted as mean with SE (N = 7; Wilcoxon test for paired data, *P < .05; NS indicates not significant [P = .14]). (C) Matured IVIg-DCs (–) or CTRL-DCs (—) were cultured for 5 days with allogeneic T cells and allogeneic NK cells from the same donor in the absence (left graph) or presence (right graph) of CD16-blocking antibody (5D2) (10 μg/mL). Addition of 5D2 restored the capacity of IVIg-DCs to stimulate allogeneic T cells (N = 6; Wilcoxon test for paired data, **P < .01, *P < .05). (D) Matured IVIg-DCs (–) or CTRL-DCs (—) were cultured for 5 days with allogeneic T cells alone (left graph) or with allogeneic T cells and autologous NK cells (right graph). In the latter case, DCs and NK cells are from the same donor. In addition, DCs were cultured with autologous NK cells and allogeneic T cells, and CD16-blocking antibody (5D2) (10 μg/mL) was added to the culture (right below graft). Addition of 5D2 restored the capacity of IVIg-DCs to stimulate allogeneic T cells (N = 3; **P < .01, *P < .05). Error bars in panels A, C, and D represent SD.
Impaired allogeneic T-cell priming is due to ADCC of IVIg-DCs by NK cells. (A) Matured CTRL-DCs, IVIg-DCs, or HSA-DCs were cultured for 18 hours with allogeneic NK cells (ratio, 1:6) with (black bars) or without (white bars) 10 μg/mL CD16-blocking antibody (5D2). DC death was determined by 7-AAD uptake. The increased death of IVIg-DCs was abrogated by blocking the FcγRIII on NK cells (N = 5; Wilcoxon test for paired data, *P < .05; NS indicates not significant). (B) Matured CTRL-DCs (□) and IVIg-DCs (■) were cultured for 18 hours with allogeneic NK cells (ratio, 1:6) with or without 10 μg/mL 5D2 antibody. Absolute numbers of viable DCs were calculated by determining the ratio of 7-AAD− DCs to detected beads and then multiplying this ratio by the number of beads in the tube. Data are depicted as mean with SE (N = 7; Wilcoxon test for paired data, *P < .05; NS indicates not significant [P = .14]). (C) Matured IVIg-DCs (–) or CTRL-DCs (—) were cultured for 5 days with allogeneic T cells and allogeneic NK cells from the same donor in the absence (left graph) or presence (right graph) of CD16-blocking antibody (5D2) (10 μg/mL). Addition of 5D2 restored the capacity of IVIg-DCs to stimulate allogeneic T cells (N = 6; Wilcoxon test for paired data, **P < .01, *P < .05). (D) Matured IVIg-DCs (–) or CTRL-DCs (—) were cultured for 5 days with allogeneic T cells alone (left graph) or with allogeneic T cells and autologous NK cells (right graph). In the latter case, DCs and NK cells are from the same donor. In addition, DCs were cultured with autologous NK cells and allogeneic T cells, and CD16-blocking antibody (5D2) (10 μg/mL) was added to the culture (right below graft). Addition of 5D2 restored the capacity of IVIg-DCs to stimulate allogeneic T cells (N = 3; **P < .01, *P < .05). Error bars in panels A, C, and D represent SD.
ADCC of IVIg-DCs by NK cells was indeed the main cause of the reduced T-cell priming capacity of IVIg-DCs. When the 5D2 antibody was added to block the FcγRIII on NK cells during cocultures of IVIg-treated DCs and allogeneic T and NK cells, the difference in allogeneic priming capacity between IVIg-DCs and CTRL-DCs was almost completely abolished (Figure 5C). Again, the impairment in T-cell priming of IVIg-DCs was not the result of allogeneic differences between DCs and NK cells, as autologous NK cells also killed IVIg-DCs from the same donor and reduced the allogeneic T-cell priming. In addition, impairment of IVIg-DC function by autologous NK cells could be abrogated by addition of the 5D2 mAb (Figure 5D). Addition of an equivalent concentration of mouse-IgG2a mAb as a control for the 5D2 blocking antibody to the cultures did not restore the impaired T-cell stimulatory capacity IVIg-DCs (data not shown).
IgG multimers, and not monomers, cause ADCC of IVIg-DCs by NK cells
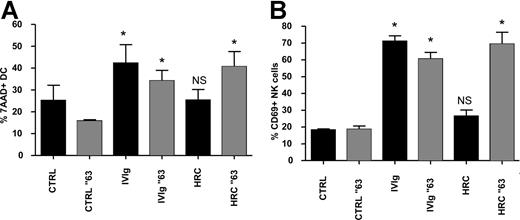
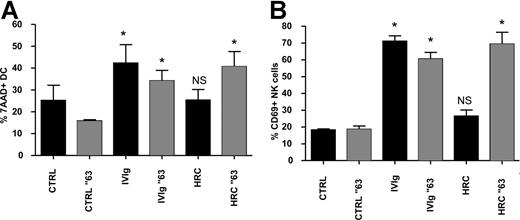
Using the fully humanized and glycosylated monoclonal antibody trastuzumab (HRC), which contains only the complementary-determining regions of a murine mAb that binds to human epidermal growth factor receptor 2,22,23 we asked whether human IgG monomers were able to promote DC lysis by NK cells. Trastuzumab treatment of maturing DCs (HRC-DCs) did not increase NK-cell activation and DC lysis in cocultures of DCs and NK cells. However, when trastuzumab was aggregated at 63°C for 30 minutes, which induces the formation of IgG aggregates,24 maturation of DCs in the presence of the aggregated form of trastuzumab promoted DC lysis by NK cells and induced NK-cell activation (Figure 6A,B). This observation suggests that the stimulation of the reciprocal interaction between DCs and NK cells by IVIgs is not mediated by IgG monomers present in the therapeutic IVIg preparations, but by IgG multimers and/or dimers.
IgG multimers, and not monomers, cause ADCC of IVIg-DCs by NK cells. (A,B) Culture medium (CTRL), IVIgs, and humanized monoclonal antibody trastuzumab (HRC) were heated at 63°C for 30 minutes (░) or left untreated (■) before addition to immature blood DCs during their maturation with proinflammatory cytokines. IVIgs and HRCs were added to the DCs in a concentration of 10 mg/mL. After 18 hours of maturation, all additions were removed, and allogeneic NK cells were added at a ratio of DC/NK of 1:6. Percentages of 7-AAD+ DCs (A) and CD69 expression on NK cells (B) were determined after 18 and 48 hours of DC-NK coculture, respectively (N = 4; Wilcoxon test for paired data, *P < .05). Error bars represent SD.
IgG multimers, and not monomers, cause ADCC of IVIg-DCs by NK cells. (A,B) Culture medium (CTRL), IVIgs, and humanized monoclonal antibody trastuzumab (HRC) were heated at 63°C for 30 minutes (░) or left untreated (■) before addition to immature blood DCs during their maturation with proinflammatory cytokines. IVIgs and HRCs were added to the DCs in a concentration of 10 mg/mL. After 18 hours of maturation, all additions were removed, and allogeneic NK cells were added at a ratio of DC/NK of 1:6. Percentages of 7-AAD+ DCs (A) and CD69 expression on NK cells (B) were determined after 18 and 48 hours of DC-NK coculture, respectively (N = 4; Wilcoxon test for paired data, *P < .05). Error bars represent SD.
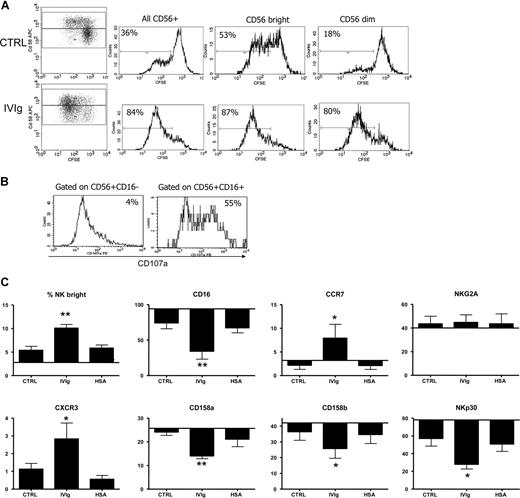
IVIg-DCs promote expansion of NK cells and induce CD56brightCD16−CCR7+CXCR3+ lymph node–type NK cells
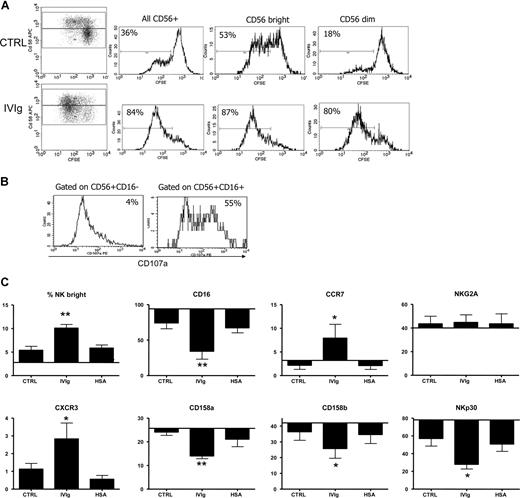
We showed that IVIg-DCs, when cocultured with allogeneic T cells and NK cells, have a decreased T-cell stimulatory capacity and that this effect is dependent on the presence of NK cells. Moreover, looking at the proliferation of NK cells in these cultures using CFSE dilution, we observed that IVIg-DCs not only activated the NK cells, but also promoted their proliferation. When IVIg-DCs were cocultured with T and NK cells, 88% (± 3%) of all CD56+ cells, 91% (± 3%) of CD56 bright cells, and 86% (± 4%) of CD56dim cells had undergone more than one round of division, compared with CTRL-DC cocultures where 48% (± 13%) of all CD56+ cells, 59% (± 5%) of CD56bright cells, and 35% (± 10%) of CD56dim started to proliferate (P < .05 for comparison of the proliferation of CD56+ NK cells, CD56bright cells, and CD56dim cells upon stimulation with IVIg-DCs compared with CTRL-DCs; N = 4; Figure 7A). Thus, while CTRL-DCs stimulated proliferation of CD56bright NK cells more strongly than proliferation of CD56dim cells, IVIg-DCs promoted similar expansion of CD56bright and CD56dim NK cells. NK-cell proliferation was not observed in cocultures of DCs and NK cells without T cells, showing that T cells, although dispensable for NK activation, were indispensable for NK-cell proliferation. To dissect differences in cytotoxicity, we harvested NK cells from the cultures, and determined CD107a expression on both CD56+CD16+ and CD56+CD16− NK-cell subtypes upon PMA/ionomycin stimulation. Indeed, a difference in CD107a expression between the 2 NK subtypes, gained after 5 days of culture with IVIg-DCs and T cells, was detected. The CD16+ subset expresses a higher level of CD107a than the CD16− NK cells after stimulation (Figure 7B). We concluded that, while IVIgs enhance the proliferation of both subsets, their cytotoxic profile is unchanged. In addition, the 5-day cocultures of NK cells with IVIg-DCs and T cells resulted in higher proportions of CD56bright NK cells in comparison with CTRL-DCs. After cultures of CTRL-DCs with T and NK cells, 5.4% (± 2%) of CD56+ cells were CD56bright, while upon culture with IVIg-DCs, 10.1% (± 2%) of the CD56+ cells were CD56bright (Figure 7C). Moreover, upon stimulation with IVIg-DCs higher percentages of NK cells expressed the lymph node homing chemokine receptors CCR7 and CXCR3. Furthermore, NKp30, which is reported to be one of the receptors involved in DC–NK-cell interaction,25 was down-regulated when NK cells were cocultured with IVIg-DCs. The expression of CD16 and of the KIR receptors CD158a and CD158b on NK cells was also reduced when NK cells are cultured with IVIg-DCs and T cells. Together, these results show that in the presence of T cells, IVIg-DCs stimulated vigorous proliferation of NK cells and promoted immunophenotypic changes resulting in an enrichment of CD56brightCD16−CCR7+CXCR3+ lymph node–type NK cells. (Figure 7C)
IVIg-DCs promote expansion of NK cells and induce CD56brightCD16−CCR7+CXCR3+ lymph node–type NK cells. (A) CTRL-DCs (top plots) or IVIg-DCs (bottom plots) were cultured with CFSE-labeled allogeneic T cells and NK cells at the ratio of DC/T/NK of 1:48:12, and after 5 days proliferation was determined. (Dotplots) The 2 top quadrants show proliferation of NK cells (CD56+CD3−); the 2 bottom quadrants show proliferation of the T cells (CD56−CD3+). (Histograms) Proliferation of all NK cells (left histogram), CD56bright NK cells (middle histogram), and CD56dim NK cells (right histogram). The plots are representative of 1 of 4 experiments. Percentages indicate proportions of NK cells that have undergone at least one division. (B) After the 5-day culture of NK cells with IVIg-DCs, NK cells were stimulated with PMA and ionomycin (2.5 μg/mL and 0.5 μg/mL), and CD107a expression on CD56+CD16+ and CD56+CD16− subsets was determined. (C) After 5 days of culture with CTLR-DCs, IVIg-DCs, or HSA-DCs, percentages of CD56bright NK cells, and percentages of NK cells expressing CD16, KIR receptors CD158a and CD158b, lymph node homing chemokine receptors CCR7 and CXCR3, and natural cytotoxicity receptors NKG2A and NKp30 were determined. NK cells cultured with IVIg-DCs up-regulated CCR7 and CXCR3, and down-regulated CD16, the KIR receptors, and NKp30 (N = 4; Student t test for paired data, *P < .05 compared with NK cells cocultured with CTRL-DCs). The percentages of cells are displayed within reference to the baseline percentage of expression of the markers on the NK cells before addition to culture. No expression of CXCR3 on NK cells was detected at this time point. Error bars represent SD.
IVIg-DCs promote expansion of NK cells and induce CD56brightCD16−CCR7+CXCR3+ lymph node–type NK cells. (A) CTRL-DCs (top plots) or IVIg-DCs (bottom plots) were cultured with CFSE-labeled allogeneic T cells and NK cells at the ratio of DC/T/NK of 1:48:12, and after 5 days proliferation was determined. (Dotplots) The 2 top quadrants show proliferation of NK cells (CD56+CD3−); the 2 bottom quadrants show proliferation of the T cells (CD56−CD3+). (Histograms) Proliferation of all NK cells (left histogram), CD56bright NK cells (middle histogram), and CD56dim NK cells (right histogram). The plots are representative of 1 of 4 experiments. Percentages indicate proportions of NK cells that have undergone at least one division. (B) After the 5-day culture of NK cells with IVIg-DCs, NK cells were stimulated with PMA and ionomycin (2.5 μg/mL and 0.5 μg/mL), and CD107a expression on CD56+CD16+ and CD56+CD16− subsets was determined. (C) After 5 days of culture with CTLR-DCs, IVIg-DCs, or HSA-DCs, percentages of CD56bright NK cells, and percentages of NK cells expressing CD16, KIR receptors CD158a and CD158b, lymph node homing chemokine receptors CCR7 and CXCR3, and natural cytotoxicity receptors NKG2A and NKp30 were determined. NK cells cultured with IVIg-DCs up-regulated CCR7 and CXCR3, and down-regulated CD16, the KIR receptors, and NKp30 (N = 4; Student t test for paired data, *P < .05 compared with NK cells cocultured with CTRL-DCs). The percentages of cells are displayed within reference to the baseline percentage of expression of the markers on the NK cells before addition to culture. No expression of CXCR3 on NK cells was detected at this time point. Error bars represent SD.
Discussion
The present study unravels the main mechanism of action by which IVIgs hamper the process of T-cell priming by DCs, that is, by inducing antibody-dependent cellular cytotoxicity (ADCC) of IVIg-treated DCs by NK cells. By binding to the DC surface, IVIgs facilitate recognition of mature DCs by NK cells via FcγRIII, resulting in activation of the NK cells. These acquire the capacity to kill the IVIg-DCs by ADCC, thereby reducing in numbers the total stimulatory pool of mature DCs. Interestingly, multimers, but not monomers of human monoclonal IgG, could recapitulate the effects of IVIgs in our culture systems, suggesting that IgG multimers and/or dimers in IVIg preparations are the active components that induce NK-cell activation and DC lysis. In addition, IVIg-DCs stimulate the proliferation of NK cells and induce CD56bright NK cells with lymph node homing properties, which can further shape the adaptive immune response.
Clinically, IVIgs have been shown to be effective for the treatment of a variety of immune-mediated inflammatory diseases,1 but still their actual mode of action on the cellular components of the human immune system remains unclear. We showed that by modulating the interaction between 2 cell types of innate immune system (ie, DCs and NK cells) IVIgs can affect the development of the linked adaptive immune response.
First, we demonstrated that IVIgs bind to the surface of maturing DCs. Using a blocking mAb for FcγRIII, we demonstrated that ADCC of IVIg-DCs occurs due to binding of the Fc-region of cell-bound IgG to FcγRIII on NK cells. More specifically, since humanized monoclonal antibody multimers, but not monomers, could recapitulate the effects of IVIgs on DC-NK interaction, death of DCs is induced upon recognition of DC-bound IgG multimers by FcγRIII on NK cells. Indeed, binding of immune complexes to FcγRIII has been reported to induce target cell lysis by NK cells, through activation via an immunoreceptor tyrosine–based activation motif.26 CD69 is an early marker for NK-cell activation and was enhanced profoundly when NK cells were cultured with IVIg-DCs. IVIg treatment of NK cells alone was not able to induce the same level of activation of NK cells. Although the matured CTRL-DCs alone were also able to activate NK cells to some extent, which is in agreement with numerous reports,12,14,27,28 only NK cells activated by IVIg-DCs secreted substantial amounts of IFN-γ and degranulated their cytotoxic granules. Therefore, both IVIgs and DCs were needed for full NK-cell activation. NK-cell activation and degranulation resulted in killing of the majority of DCs matured in the presence of IVIgs, which could be largely abrogated by FcγRIII blockade. Therefore killing of IVIg-DCs is predominantly attributable to ADCC mediated by activated NK cells. Since death of IVIg-DCs was not completely abrogated by CD16 blockade, additional cytotoxic mechanisms may contribute to the profound killing of IVIg-DCs in the presence of NK cells (eg, cytotoxic effect of IFN-γ produced by the NK cells).29
So far to our knowledge, this phenomenon has not been described as one of the potential modes of action of IVIgs on the cellular immune system. Supporting our finding, administration of high doses of IVIgs in patients with Kawasaki disease has been reported to increase the numbers of circulating NK cells and to elevate ADCC activity of NK cells.30 However, this does not exclude that other mechanisms may also contribute to the impaired T-cell stimulatory capacity of IVIg-treated DCs. In a previous study, we observed that IVIg-treated blood DCs, when cultured with T and NK cells, were suppressed in their up-regulation of costimulatory molecules CD40 and CD80.9 In addition, IVIgs have been shown to suppress differentiation of human monocytes to DCs, and to inhibit maturation of monocyte-derived DCs.8 Moreover, IVIgs have been shown to induce regulatory activity in murine DCs via activating Fcγ-receptors.10 However, according to the dramatic effect of CD16 blockade on the T-cell stimulatory capacity of IVIg-DCs (Figure 5C,D), we reckon that NK-mediated DC killing is the most important mechanism of action by which IVIgs reduce the T-cell stimulatory capacity of DCs.
In vivo NK cells interact with DCs in lymph nodes and in inflamed peripheral tissues,13,31-35 and the conditions in which DCs and NK cells encounter one another can determine the following T-cell responses.12-14 Equipped to perform “DC editing,” NK cells can induce killing of immature DCs that fail to undergo proper maturation. Since mature DCs are resistant to lysis by NK cells, this is believed to provide help to create fully potent adaptive responses.32,36 However, while performing their DC editing task, NK cells may, upon IVIg therapy, also recognize the mature IVIg-DC as a target cell, as it is fully coated with IgG. In this case, the NK cell may kill the IVIg-coated mature DCs, and thereby reduce the pool of immunogenic antigen-presenting cells migrating from inflamed tissue to the lymph node, or present in the lymph node, and thereby dampen the subsequent T-cell responses.
It is intriguing that our own IgG exerts predominantly proinflammatory properties (eg, as inducer of complement- or cell-mediated cytotoxicity), while high-dose therapeutic IgG treatment has anti-inflammatory effects. Our finding that humanized monoclonal IgG caused NK-cell–mediated ADCC of DCs only after heat aggregation may elucidate this discrepancy. We propose that the active anti-inflammatory components in IVIgs are IgG multimers and dimers, which are not present in human serum from one individual, but are formed in plasma pools due to idiotype–anti-idiotype complex formation between IgG molecules from different individuals.3,4 This hypothesis is supported by studies that described IgG polymers in commercial IVIg preparations to be the active component in various immune-mediated disease models.4,37
In addition, during normal physiological immune responses IgG immune complexes can be formed, which may also stimulate NK-cell–mediated lysis of DCs, thereby inhibiting T-cell activation. In this line of thinking, accumulating evidence supports an immune regulatory role of immune complexes in physiological immune responses. In 2 different IVIg-treatable autoimmune disease models in rodents (ie, ITP and arthritis), Siragam et al demonstrated that both small soluble and large particulate immune complexes can mimic the therapeutic effects of IVIg treatment.38 Recently, it was shown that IgG immune complexes induce an IFN-γ refractory state in macrophages via binding to FcγRIII on their surface, thereby providing a potential mechanism for the immunosuppressive effects of immune complexes in IFN-γ–driven inflammatory responses.39
Human CD56brightCD16− NK cells (NKbright) and CD56dimCD16+ NK cells (NKdim) are reported to have distinct functions. The NKdim subset exhibits primarily a cytotoxic effector function, whereas NKbright cells can produce immunoregulatory cytokines such as IFN-γ, tumor necrosis factor β (TNF β), IL-10, IL-13, and GM-CSF.40,41 Surprisingly, IVIg-DCs stimulated immunophenotypic changes in NK cells resulting in an increased proportion of NKbright cells, with reduced expression of CD16, NKp30, and the KIR receptors CD158a and CD158b, and enhanced expression of the chemokine receptors CCR7 and CXCR3, which can mediate NK-cell migration to secondary lymphoid organs.31,42,43 Together, these immunophenotypic changes confirm the induction of lymph node–type NK cells.31,44,45 To date, the mechanism of action by which IVIg-DCs promote enrichment of the NKbright population is not understood. Since IVIg-DCs stimulate proliferation of NKdim and NKbright cells to a similar extent, the enrichment of NKbright cells is not due to differential expansion. Most likely, it is due to differentiation from NKdim cells. NKbright cells maintain interactions with DCs in lymph nodes for extended periods and are not cytotoxic, but have immunomodulatory functions.13,34,46 Induction of NKbright cells may, therefore, constitute a mechanism of action by which immunomodulatory effects of IVIg treatment remain after IVIgs have been cleared.
Therefore, we speculate that in vivo IVIg treatment exerts its effect on cellular immunity by affecting NK-DC cross talk. An immediate immunosuppressive effect is caused by promoting the interaction between cytotoxic NKdim cells and DCs resulting in killing of mature DCs. This is expected to occur mainly in inflamed nonlymphoid tissues.35 Recent evidence confirms that NK cells can prevent immune activation in vivo. NK cells regulated T-cell priming in a skin transplant model by killing donor antigen-presenting cells,47 and NK-cell alloreactivity after hematopoietic stem cell transplantation from unrelated donors is associated with decreased incidence of graft-versus-host disease.48,49
In light of our findings, we propose a model to clarify how administration of IVIgs may dampen hyperactivity of the cellular immune system for extended periods. IgG multimers and dimers present in IVIg preparations stimulate NK-cell–mediated ADCC of mature DCs, and thereby prevent proper T-cell priming. This model also has physiological relevance, in that it predicts that immunoglobulin-antigen immune complexes may contribute to the termination of the T-cell responses during clearance of infections and to maintaining tolerance to self-antigens. As immune complexes emerge at the end of the humoral responses, physiological immune complexes as well as immune complexes in IVIg preparations may be the essential element that contributes to the termination or “cooling down” of the immune system after prolonged activation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Mosaic grant of the Netherlands Organization for Scientific Research (NWO 017.002.036) to T.T., and by an unrestricted grant of Biotest Seralc (Soest, the Netherlands) to J.K.
The authors would like to acknowledge Prof K. J. Wood (Nuffield Department of Surgery, Oxford) for review of the paper and helpful comments, Dr M. de Haas (Sanquin Research) for providing the clone 5D2 mAb, and Dr P. J. S. van Kooten (Faculty of Veterinary Medicine, Utrecht University) for assistance in biotinylating IVIgs.
Authorship
Contribution: T.T. performed the experiments, analyzed the data, and wrote the paper; H.J.M. designed the research and provided clinical input; H.W.T. and E.J.K. provided clinical input; Z.M.A.G. performed the experiments; R.A.M. contributed to study design; J.K. designed and supervised the research and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaap Kwekkeboom, Laboratory of Gastroenterology and Hepatology, Rm L-455, Erasmus MC–University Medical Center, 3015 CE Rotterdam, the Netherlands; e-mail: j.kwekkeboom@erasmusmc.nl.

![Figure 1. Impaired allogeneic T-cell priming by IVIg-DCs occurs only in the presence of NK cells. (A) Indicated numbers of immature blood DCs were stimulated with TNFα and IL-1β for 18 hours in the absence (CTRL-DC, —) or presence of 10 mg/mL IVIgs (IVIg-DC, –). The next day, DCs were washed, and allogeneic T cells, which contained 12% NK cells, or the same number of allogeneic T cells without NK cells were added. Proliferation was assessed after 5 days by determination of the incorporation of [3H] thymidine (N = 4; *P < .05, **P < .01, paired Student t test). Error bars are SD. (B) Purified allogeneic T cells and NK cells labeled with CFSE were added and subsequently cultured with CTRL-DCs (left) or IVIg-DCs (right) at a DC/T/NK ratio of 1:48:12. At day 6 of coculture, cells were labeled with CD3-PE and CD56-APC, and the CFSE dilution profile of CD3+CD56− T cells was analyzed using ModFit software. CFSE analysis was also performed on CD56+CD3− NK cells, which is shown in Figure 7A separately. (C) The same experiment, as depicted in panel B, but CFSE-labeled allogeneic T cells without NK cells, were cultured with CTRL-DCs (left) or IVIg-DCs (right) at a DC/T ratio of 1:48. Proliferation index (PI) and percentage of precursor T cells (% Prec) depicted in panels B,C are means plus or minus SD from 4 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/9/10.1182_blood-2007-03-077057/7/m_zh80230709340001.jpeg?Expires=1767774346&Signature=3BgQyEDTvOV5amamfWh~rf3GND9GS1Kecmcsmj2yFAgJBx1rEVhnhbZSFjDM6sUCYN-NLaEMoDqUB7CJ9KHIVrriSh~6w1HsA7kS8bRULq4G0EmN6RFTzHMKjMt8XaiZPenQ7FrT8LP8BrHWnfi0j0jsoYGzfdYSYULkqNSLLcOtObpsdfzgVvNaLm7VLo3MG2tWnj85AepH6CU-Lf7qCgaOUhwbUoUR~AFNlUSLMtMvoOffOAOfv~WZ60m6xcDg-vDE46az0J6vGHDfJBcNi8FgEFleGRtRWc-PUrkGy0wx8iF9tDJ8vfICisflsUuzkI6iV-GEPrin2sopBXJt~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Impaired allogeneic T-cell priming is due to ADCC of IVIg-DCs by NK cells. (A) Matured CTRL-DCs, IVIg-DCs, or HSA-DCs were cultured for 18 hours with allogeneic NK cells (ratio, 1:6) with (black bars) or without (white bars) 10 μg/mL CD16-blocking antibody (5D2). DC death was determined by 7-AAD uptake. The increased death of IVIg-DCs was abrogated by blocking the FcγRIII on NK cells (N = 5; Wilcoxon test for paired data, *P < .05; NS indicates not significant). (B) Matured CTRL-DCs (□) and IVIg-DCs (■) were cultured for 18 hours with allogeneic NK cells (ratio, 1:6) with or without 10 μg/mL 5D2 antibody. Absolute numbers of viable DCs were calculated by determining the ratio of 7-AAD− DCs to detected beads and then multiplying this ratio by the number of beads in the tube. Data are depicted as mean with SE (N = 7; Wilcoxon test for paired data, *P < .05; NS indicates not significant [P = .14]). (C) Matured IVIg-DCs (–) or CTRL-DCs (—) were cultured for 5 days with allogeneic T cells and allogeneic NK cells from the same donor in the absence (left graph) or presence (right graph) of CD16-blocking antibody (5D2) (10 μg/mL). Addition of 5D2 restored the capacity of IVIg-DCs to stimulate allogeneic T cells (N = 6; Wilcoxon test for paired data, **P < .01, *P < .05). (D) Matured IVIg-DCs (–) or CTRL-DCs (—) were cultured for 5 days with allogeneic T cells alone (left graph) or with allogeneic T cells and autologous NK cells (right graph). In the latter case, DCs and NK cells are from the same donor. In addition, DCs were cultured with autologous NK cells and allogeneic T cells, and CD16-blocking antibody (5D2) (10 μg/mL) was added to the culture (right below graft). Addition of 5D2 restored the capacity of IVIg-DCs to stimulate allogeneic T cells (N = 3; **P < .01, *P < .05). Error bars in panels A, C, and D represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/9/10.1182_blood-2007-03-077057/7/m_zh80230709340005.jpeg?Expires=1767774346&Signature=IjDmIEA5BjHnT2c0GRsMdYlHSAqewwCkSb3Ea7gwzX~3-y6TQCIMPZAt4Or7dUhC-voEdSFElZLbRTGR35RbRE9A44lg-y~S05IGQzDvw112bqQPi-DJUSLvfF1vMc7kzAKhzmgYjrHGBIpCtzFD6LEVZT5GsgYwkRcXYJ9ntFCTd7mul3nPMT2MjwV6zypcQT0oR5zMxWsuNjCjz2hdaGn2zO~hRhtb5kwRZ4gnq8hvEvThOvhbDAFwQatMKd6058wq9DCCM5lVcM8MjJcIw0cd3S9QyZ~eu37P48Z1WRUOhllFyVFjW7AkZwXNa7FThrpT2sU3BxizJqI3BObdGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Impaired allogeneic T-cell priming by IVIg-DCs occurs only in the presence of NK cells. (A) Indicated numbers of immature blood DCs were stimulated with TNFα and IL-1β for 18 hours in the absence (CTRL-DC, —) or presence of 10 mg/mL IVIgs (IVIg-DC, –). The next day, DCs were washed, and allogeneic T cells, which contained 12% NK cells, or the same number of allogeneic T cells without NK cells were added. Proliferation was assessed after 5 days by determination of the incorporation of [3H] thymidine (N = 4; *P < .05, **P < .01, paired Student t test). Error bars are SD. (B) Purified allogeneic T cells and NK cells labeled with CFSE were added and subsequently cultured with CTRL-DCs (left) or IVIg-DCs (right) at a DC/T/NK ratio of 1:48:12. At day 6 of coculture, cells were labeled with CD3-PE and CD56-APC, and the CFSE dilution profile of CD3+CD56− T cells was analyzed using ModFit software. CFSE analysis was also performed on CD56+CD3− NK cells, which is shown in Figure 7A separately. (C) The same experiment, as depicted in panel B, but CFSE-labeled allogeneic T cells without NK cells, were cultured with CTRL-DCs (left) or IVIg-DCs (right) at a DC/T ratio of 1:48. Proliferation index (PI) and percentage of precursor T cells (% Prec) depicted in panels B,C are means plus or minus SD from 4 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/9/10.1182_blood-2007-03-077057/7/m_zh80230709340001.jpeg?Expires=1767774347&Signature=uK-ZhCJLh5h-U3HxFFL8RclqwpbhMO0pQTNwXFwzNK5G4K3w28u2RZPrO21EvU0KIdMlDBp6-1sAhnna48OxQB4JHlYiAT7EUS32PKL1So1nWzT7uYmxbC89xhQtoSMk6xMw91yTQOZwsSe4bpjQigLxV6Ti7k5eeSeQmpqQLORerPS-TQ6ZyM-SZfR2pifGI6jhiDV0TEDkwT34gr1HR9pHxTqAoInXCy36MQXCd~z1UXZxZLL0mnuDPMvDgPp4sZ-UPT5ABXFIBSzDvHopvuiFcQrF424boRYyZGeTEz11kpI-fdaslcxeHTk2c2G6bqPxPJPPs2n1yPAkdY-dog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Impaired allogeneic T-cell priming is due to ADCC of IVIg-DCs by NK cells. (A) Matured CTRL-DCs, IVIg-DCs, or HSA-DCs were cultured for 18 hours with allogeneic NK cells (ratio, 1:6) with (black bars) or without (white bars) 10 μg/mL CD16-blocking antibody (5D2). DC death was determined by 7-AAD uptake. The increased death of IVIg-DCs was abrogated by blocking the FcγRIII on NK cells (N = 5; Wilcoxon test for paired data, *P < .05; NS indicates not significant). (B) Matured CTRL-DCs (□) and IVIg-DCs (■) were cultured for 18 hours with allogeneic NK cells (ratio, 1:6) with or without 10 μg/mL 5D2 antibody. Absolute numbers of viable DCs were calculated by determining the ratio of 7-AAD− DCs to detected beads and then multiplying this ratio by the number of beads in the tube. Data are depicted as mean with SE (N = 7; Wilcoxon test for paired data, *P < .05; NS indicates not significant [P = .14]). (C) Matured IVIg-DCs (–) or CTRL-DCs (—) were cultured for 5 days with allogeneic T cells and allogeneic NK cells from the same donor in the absence (left graph) or presence (right graph) of CD16-blocking antibody (5D2) (10 μg/mL). Addition of 5D2 restored the capacity of IVIg-DCs to stimulate allogeneic T cells (N = 6; Wilcoxon test for paired data, **P < .01, *P < .05). (D) Matured IVIg-DCs (–) or CTRL-DCs (—) were cultured for 5 days with allogeneic T cells alone (left graph) or with allogeneic T cells and autologous NK cells (right graph). In the latter case, DCs and NK cells are from the same donor. In addition, DCs were cultured with autologous NK cells and allogeneic T cells, and CD16-blocking antibody (5D2) (10 μg/mL) was added to the culture (right below graft). Addition of 5D2 restored the capacity of IVIg-DCs to stimulate allogeneic T cells (N = 3; **P < .01, *P < .05). Error bars in panels A, C, and D represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/9/10.1182_blood-2007-03-077057/7/m_zh80230709340005.jpeg?Expires=1767774347&Signature=uUn5dmoSlCWLc~~PnBjUON1jbF0C3B82NfwH9XcFrop7b2FCqCPxlGDqccaU-eRulswDqRjmPgmmPYRWWe8dNgUV3yIheL-c4XxMxBw6g-LvapAlmdA5IAm2hOelceQF8Q4~~LM-odJzXJWDcnzetC3YGarzekX-kd74v7POCH413h3cCduFUJLlA9V62FuXHJX967lln~SvCZEnGoSYRZaW0ISJJyupnvqR3iyZk6j87AaxYjX8q123~sR2YXu7tIQoRE4J4Y4gbRG5L0sI901EIVcq-tRpo0znLhprg3UFXmRoNy9y2pDsLGvJLq6Jcnhfd9wwTrbYdNmmRCmmZw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)